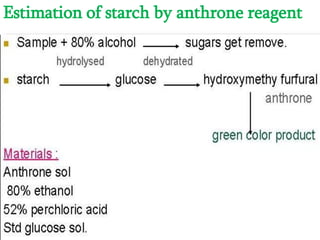
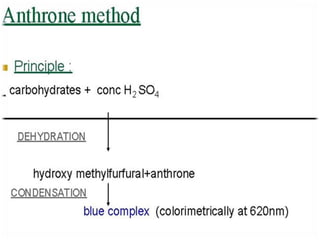
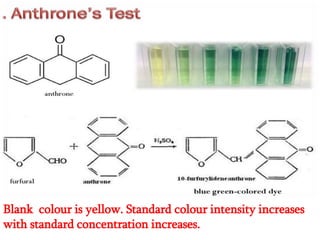
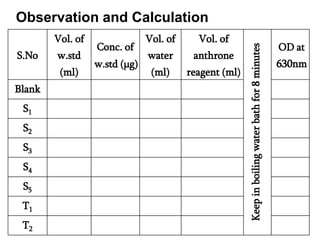
The document discusses the estimation of starch using the anthrone reagent method, highlighting starch's role as a vital polysaccharide and its hydrolysis into simple sugars. It outlines a procedure for extracting starch from samples and detailing colorimetric measurement processes, emphasizing the necessary chemical reactions and concentrations. Additionally, it provides materials needed, calculated nutritional values, and references for further reading.