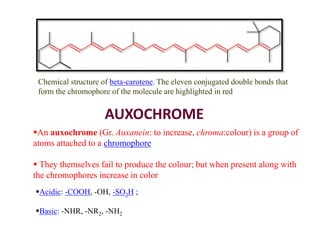
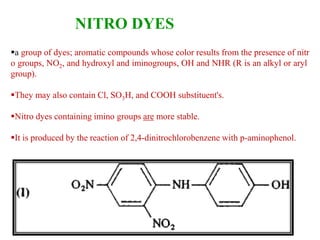
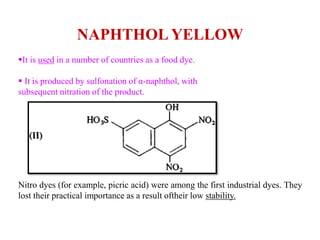
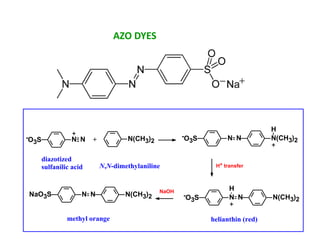
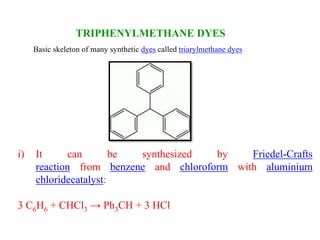
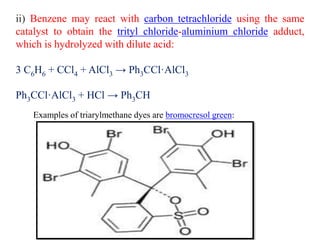
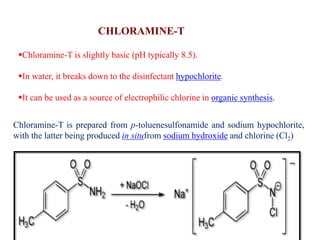
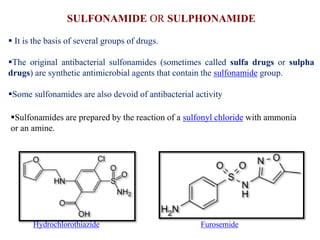
This document discusses the classification and synthesis of dyes. It begins by defining key concepts like chromophores, which are the parts of molecules responsible for color, and auxochromes, which increase the color intensity. It then describes several classes of dyes in more detail, including nitro dyes, azo dyes like methyl orange, triarylmethane dyes like malachite green, indigo dyes, anthraquinone dyes like alizarin, and fluorescein dyes. Other topics covered include benzenesulfonic acid, saccharin, chloramine-T, and sulfonamides. The document provides examples and reaction mechanisms to illustrate the synthesis of dyes from various