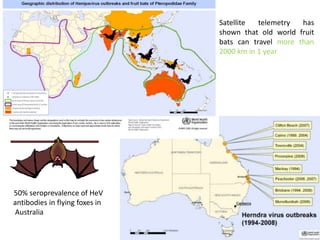
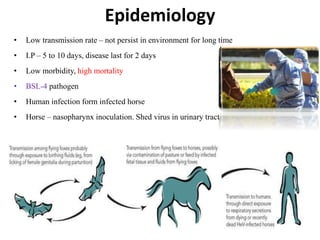
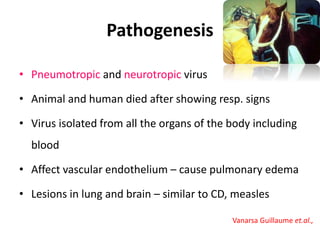
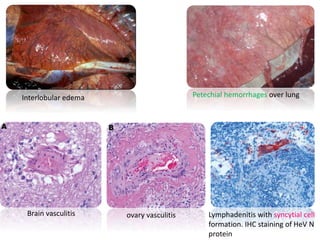
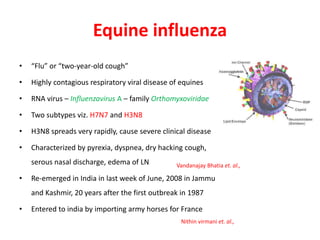
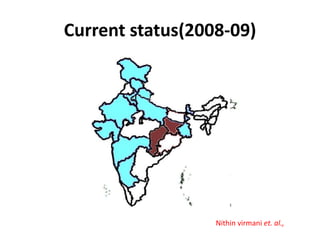
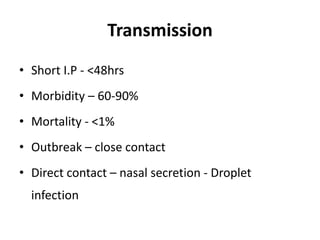
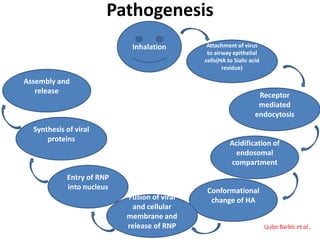
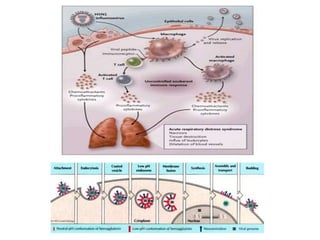
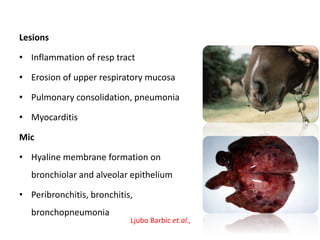
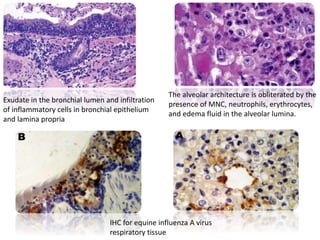
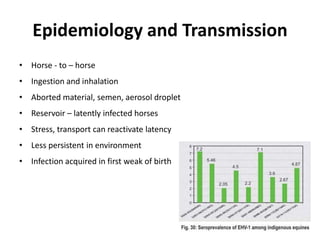
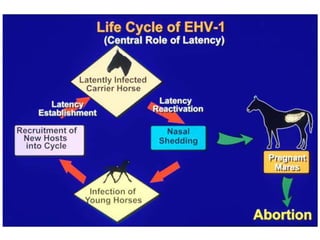
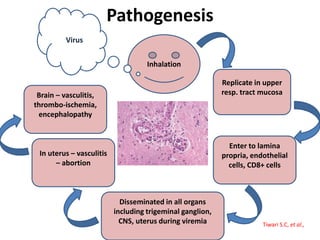
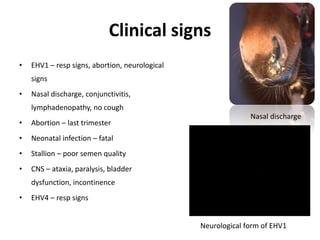
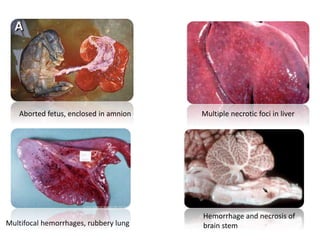
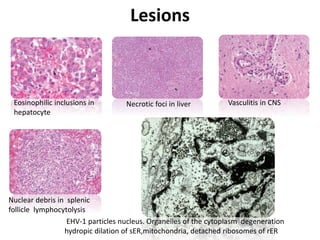
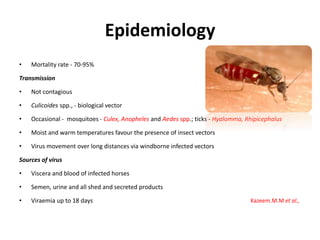
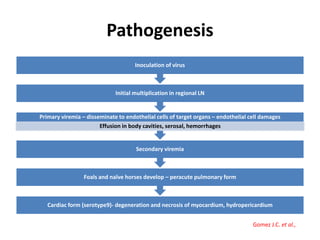
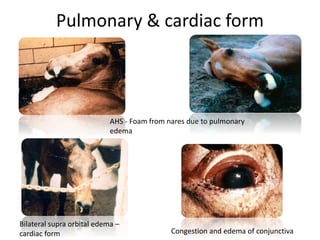
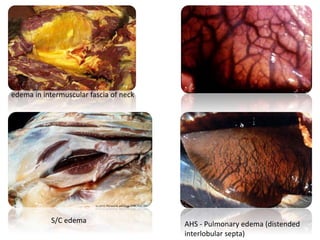
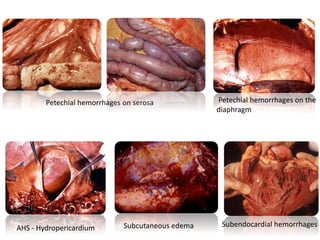
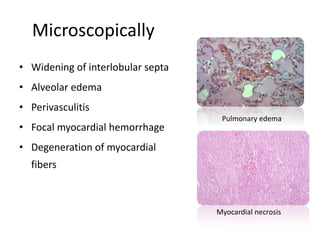
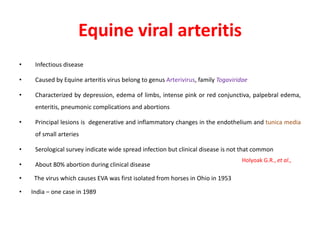
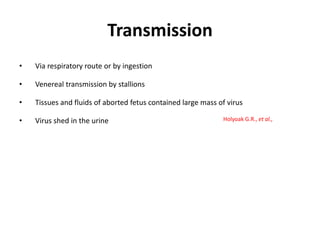
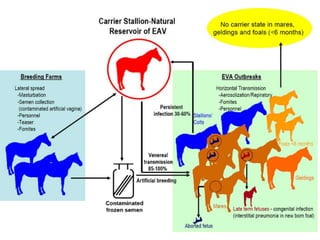
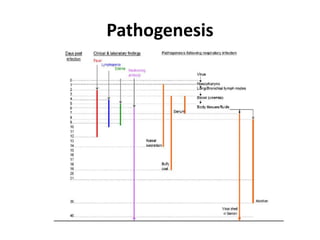
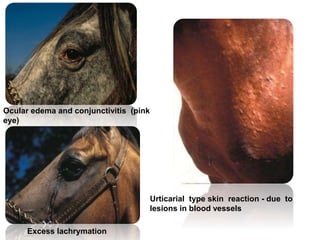
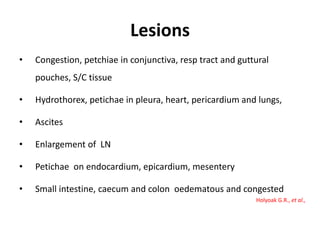
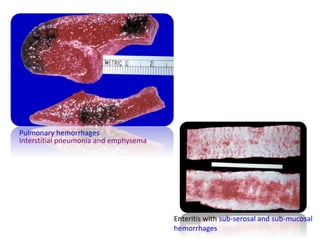
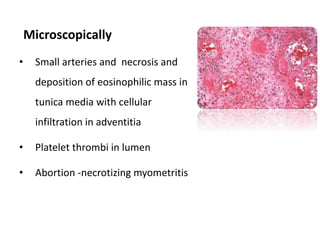
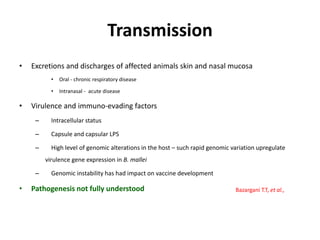
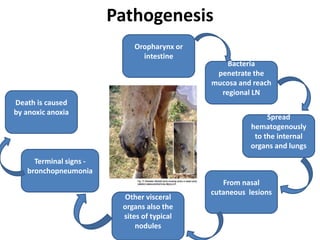
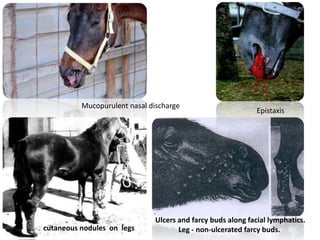
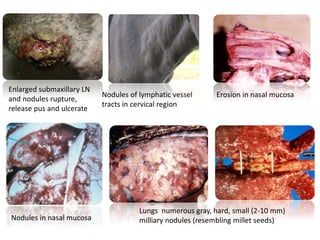
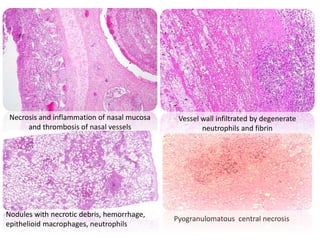
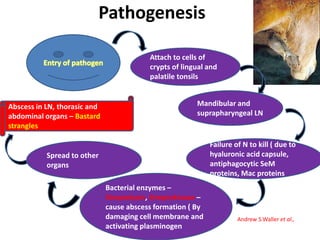
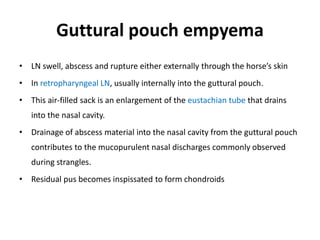
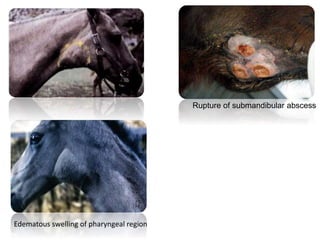
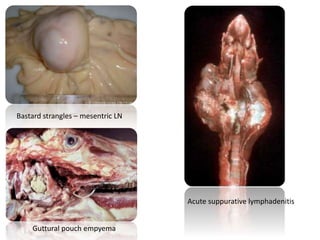
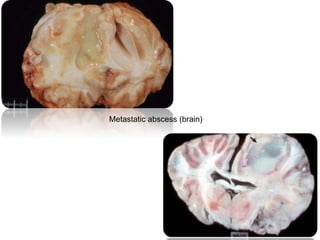
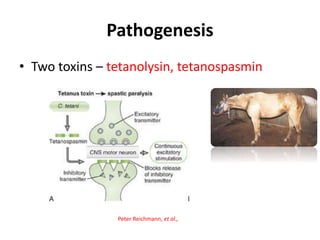
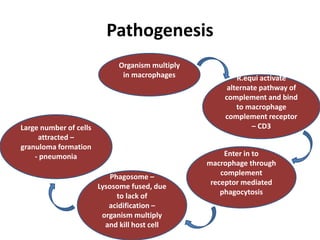
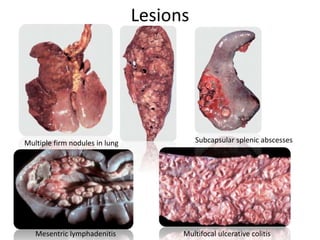
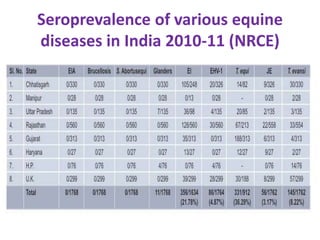
This document provides information on various diseases that affect equines in India. It begins with background on the equine population in India and then lists and describes several important viral diseases (Hendra, equine influenza, equine herpes virus, equine infectious anemia, African horse sickness, equine viral arteritis, West Nile fever, equine encephalitis) and bacterial diseases (glanders, strangles, tetanus, Rhodococcus equi, leptospirosis, botryomycosis). For each disease, it discusses the causative agent, transmission, pathogenesis, clinical signs, lesions, and current status or outbreaks in India. Considerable detail is provided for Hendra virus, equ