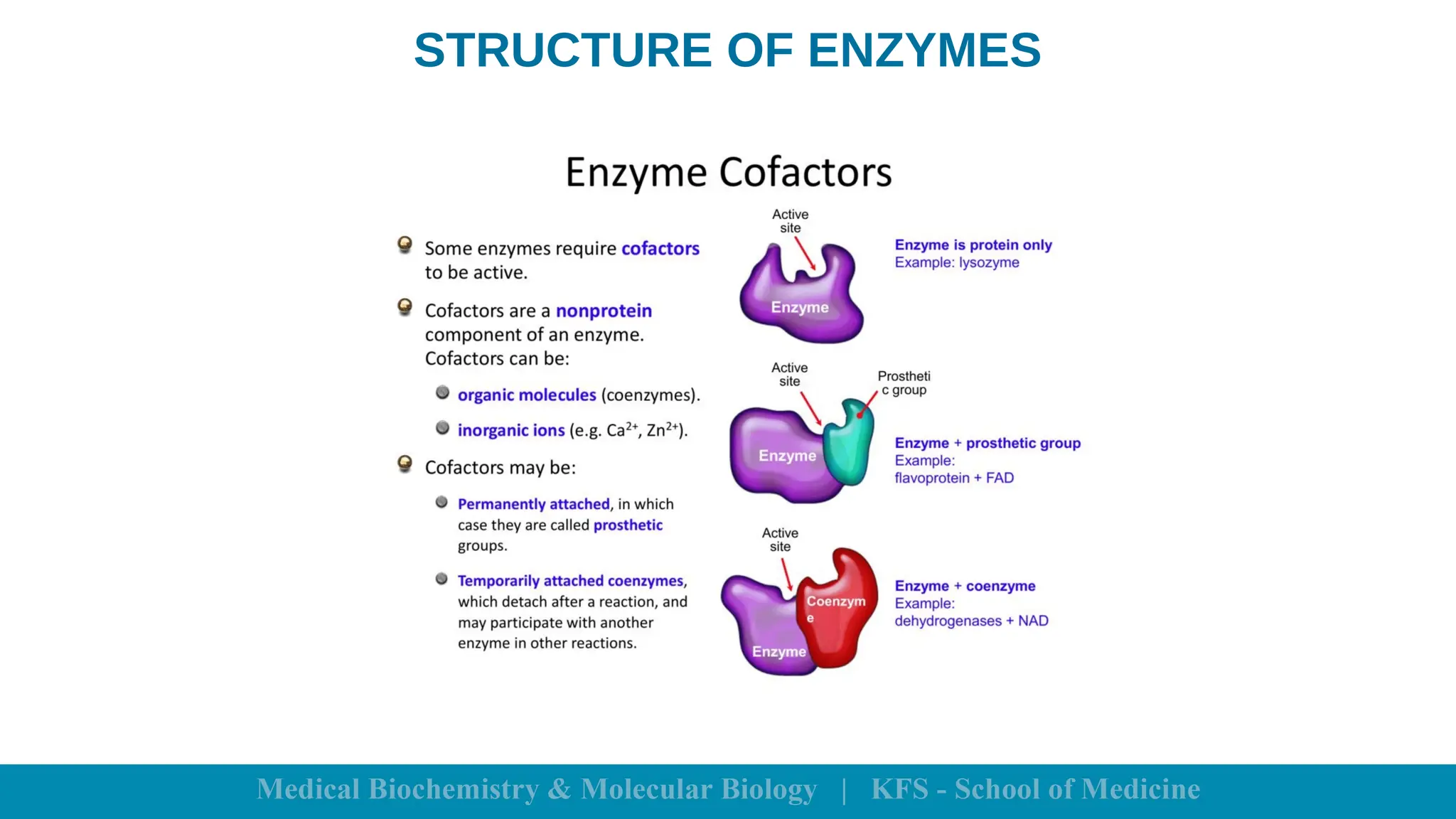
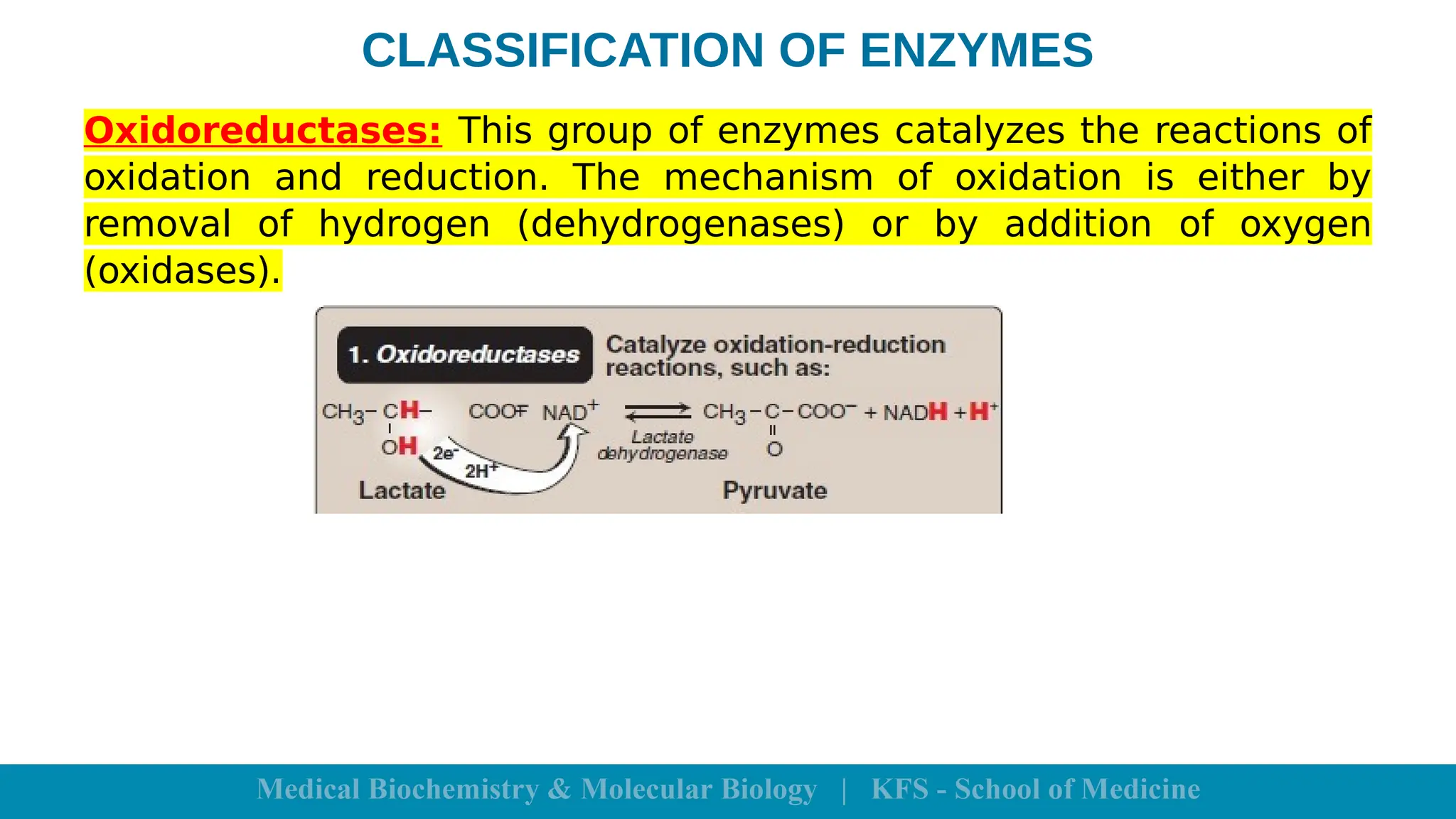
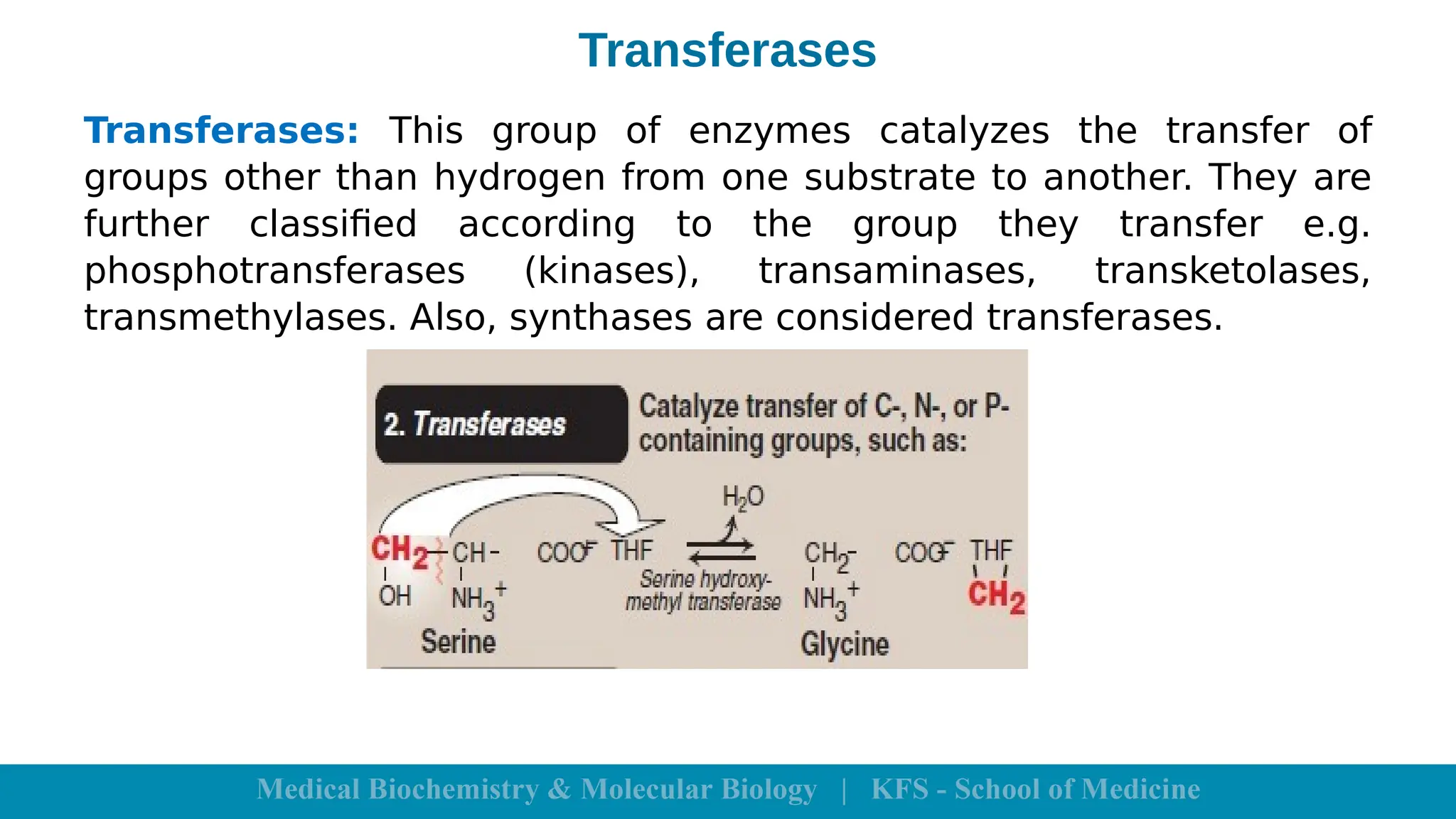
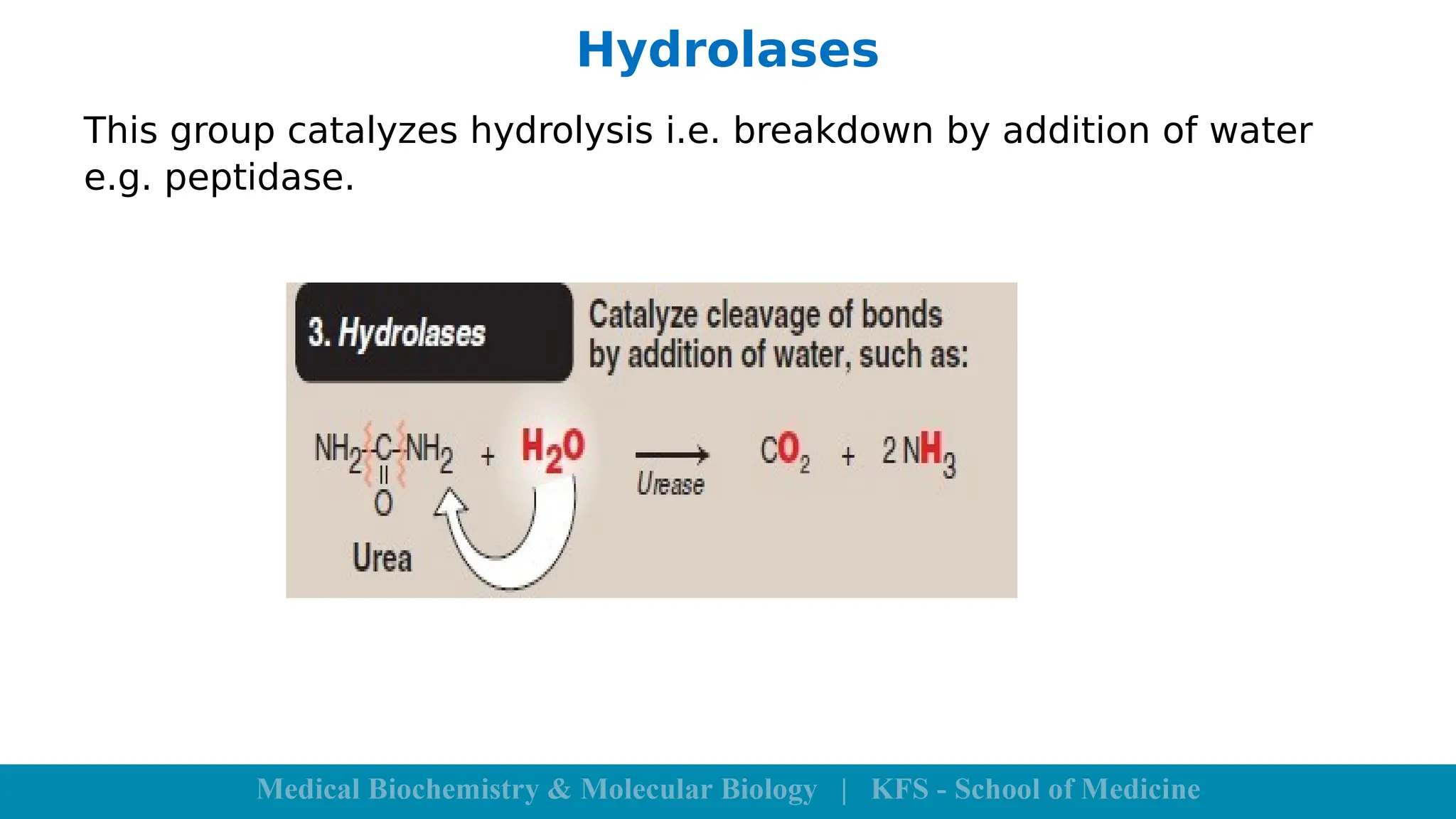
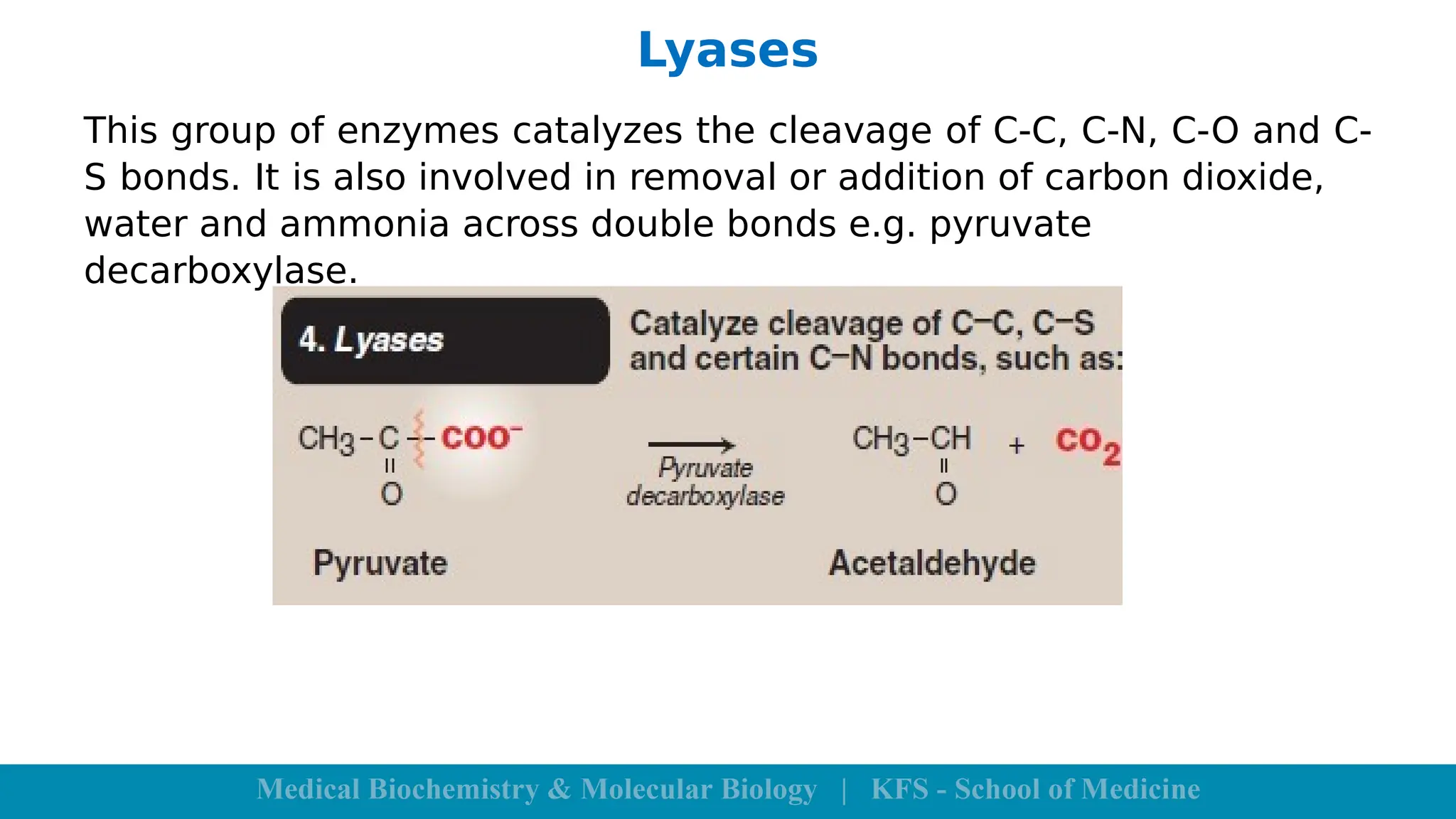
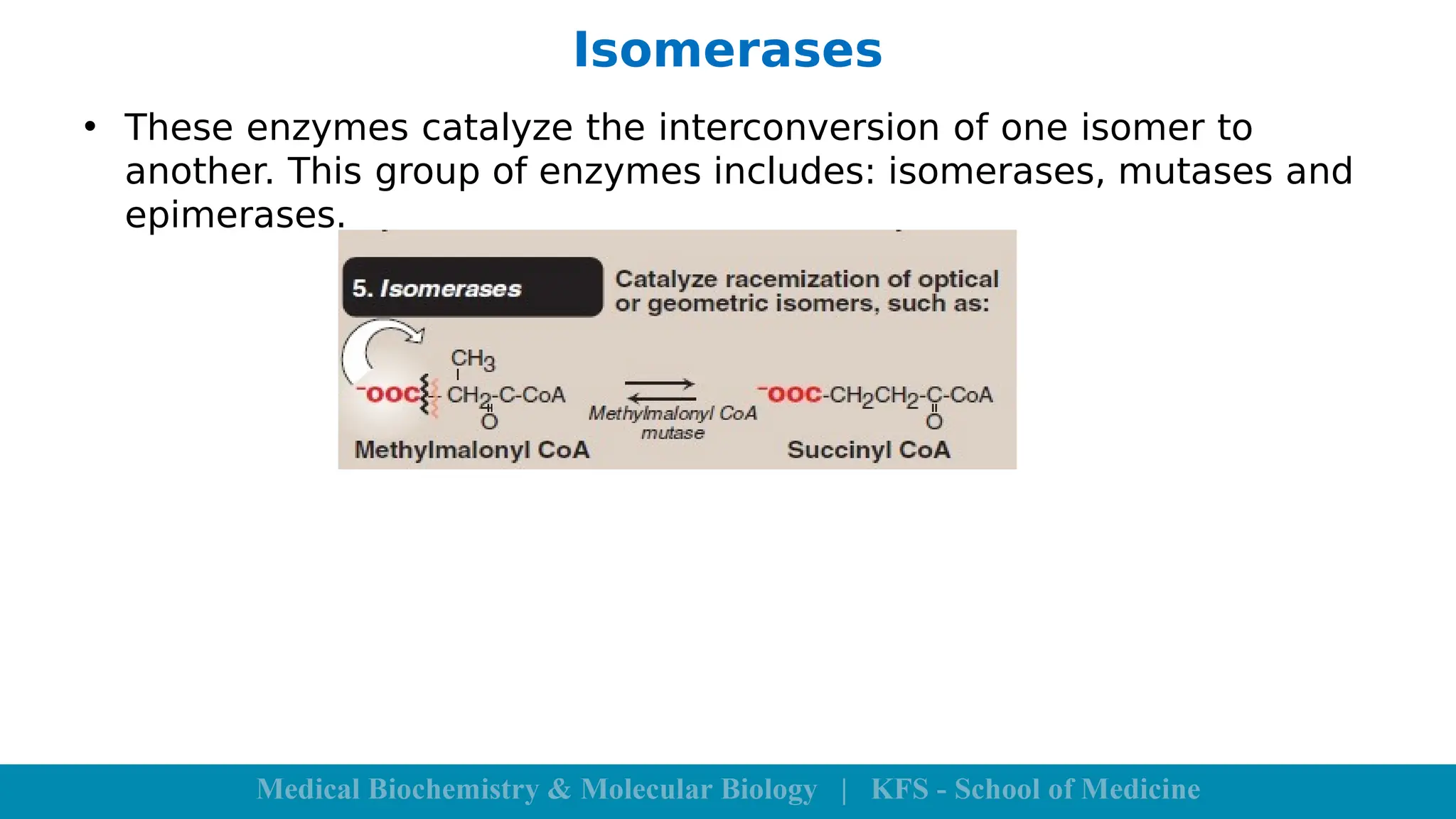
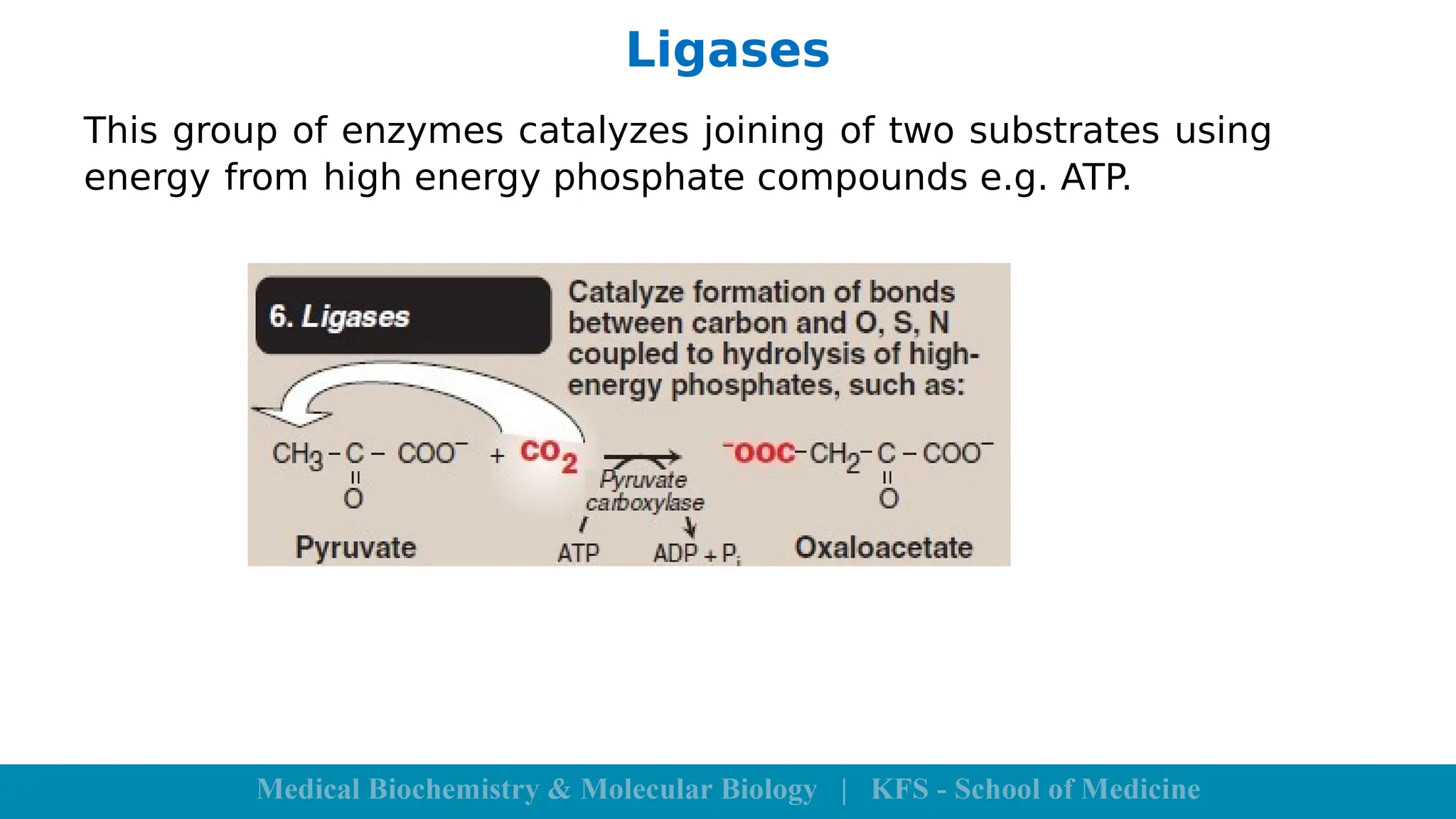
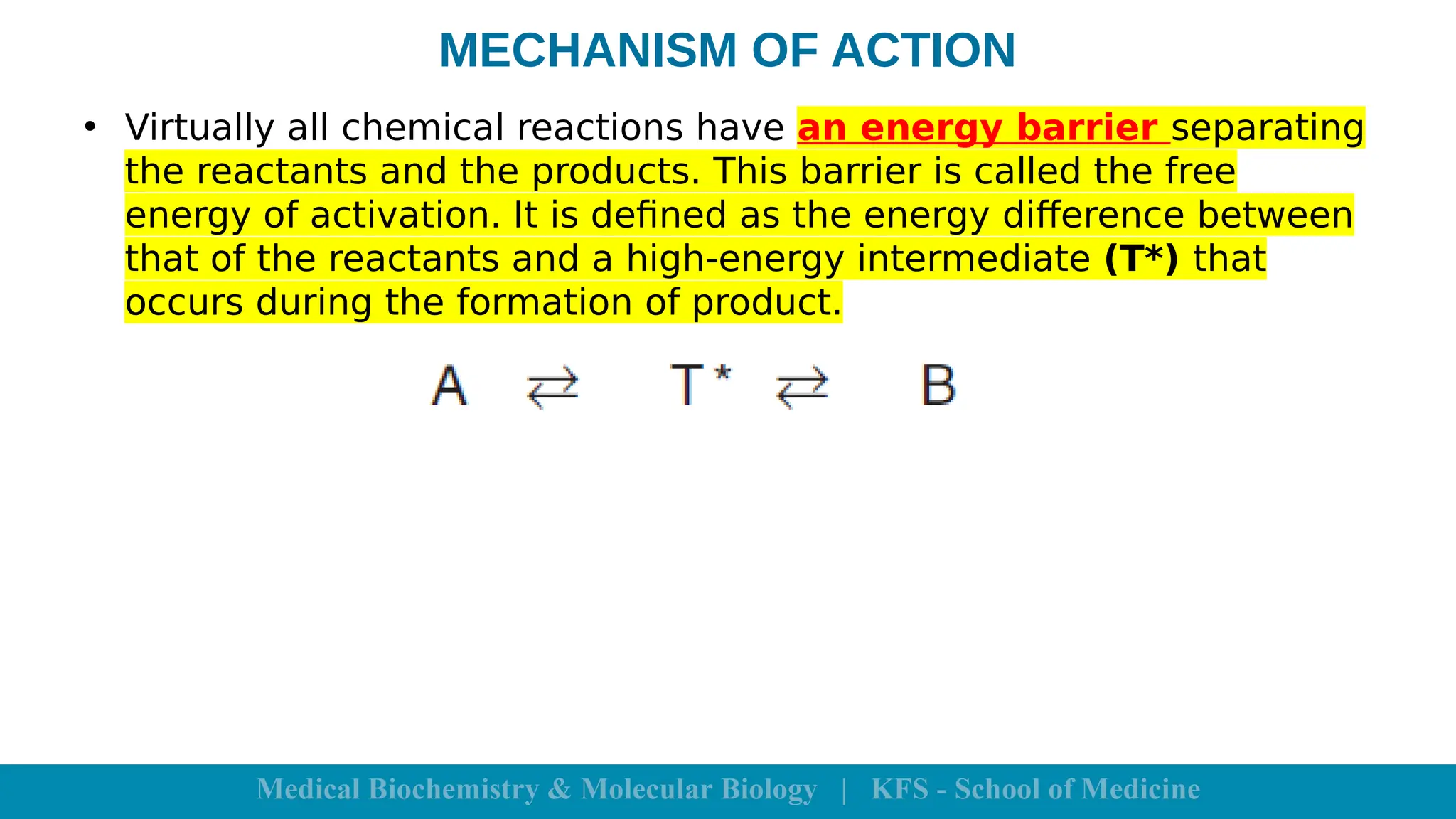
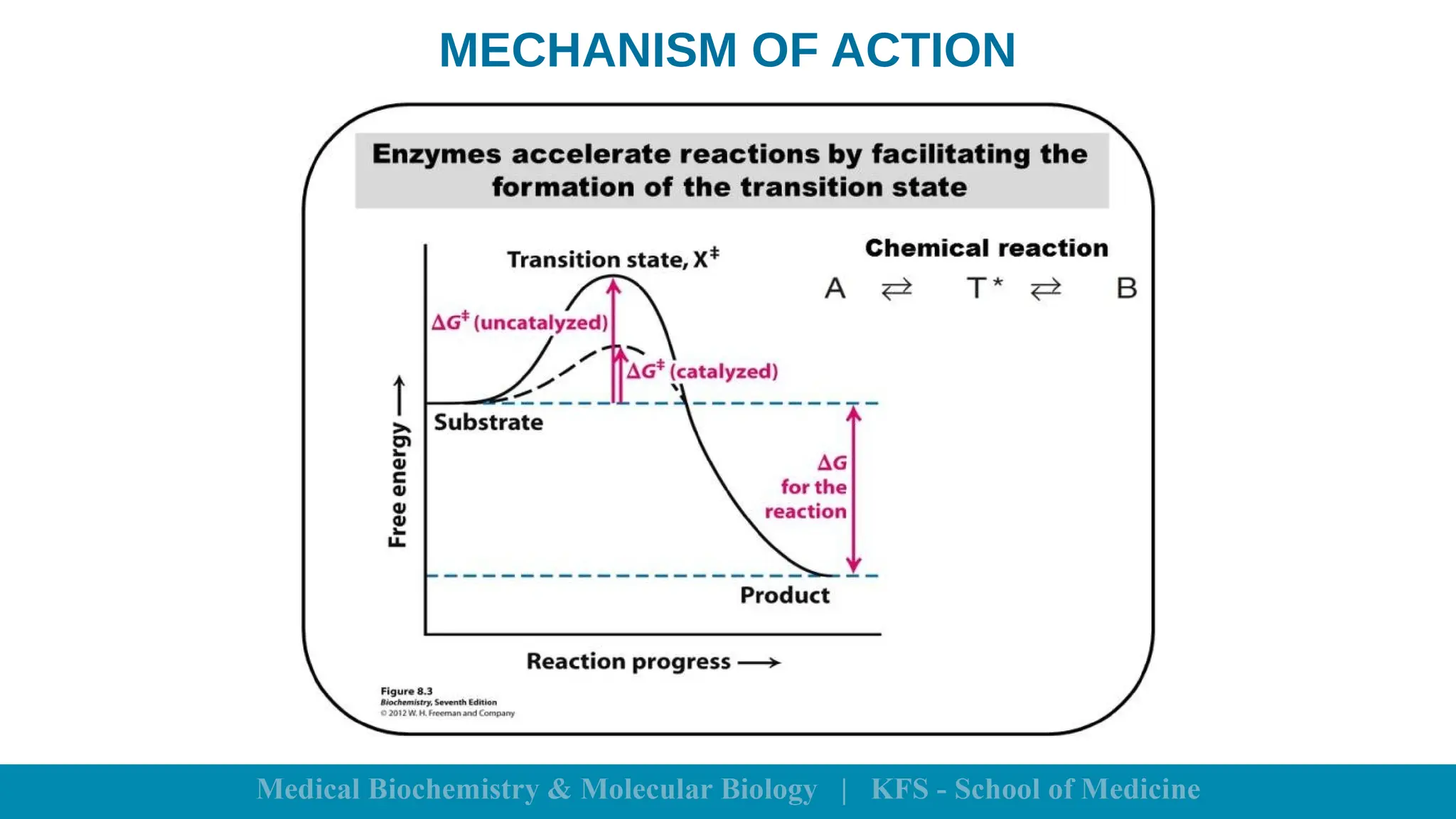
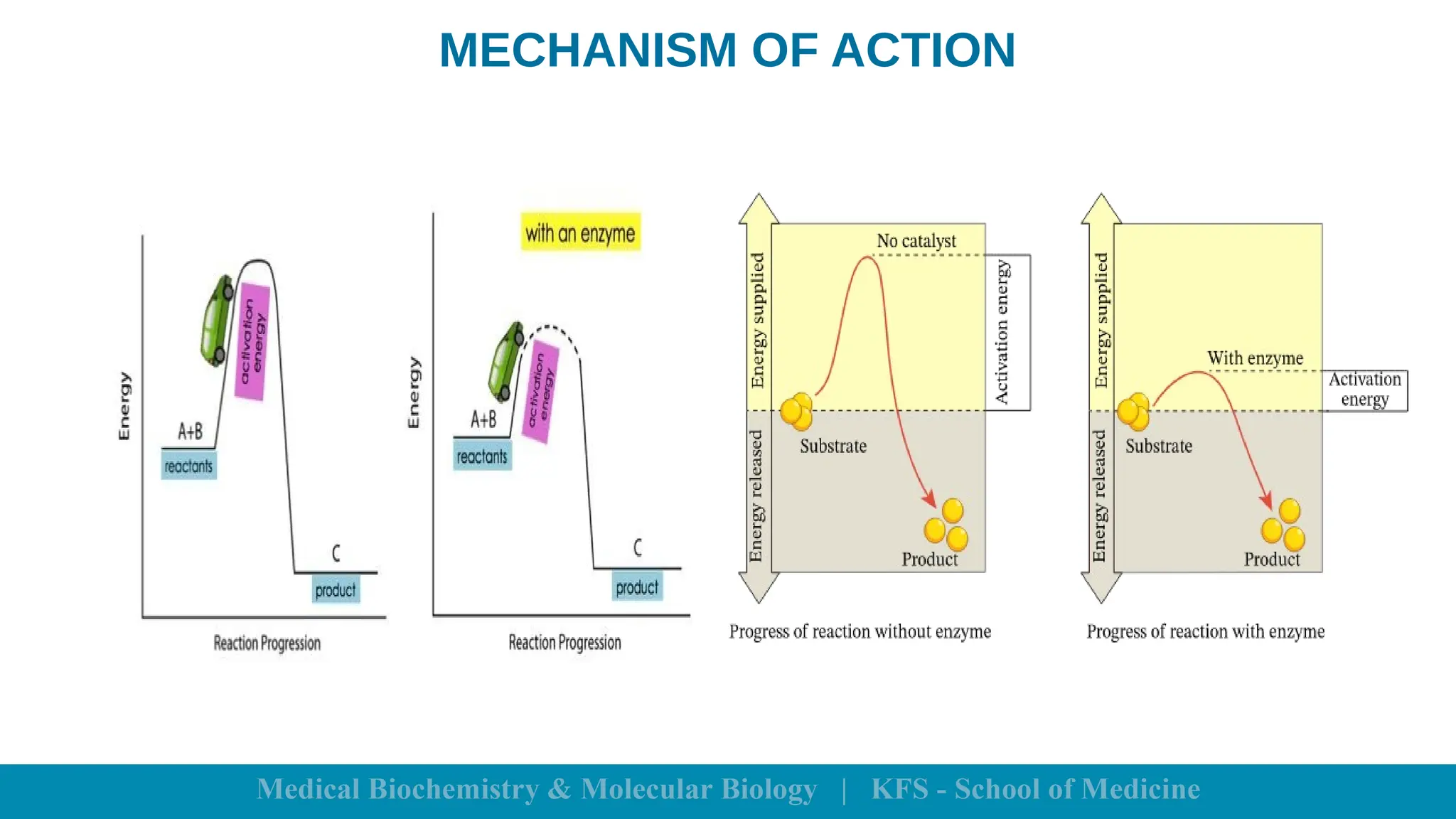
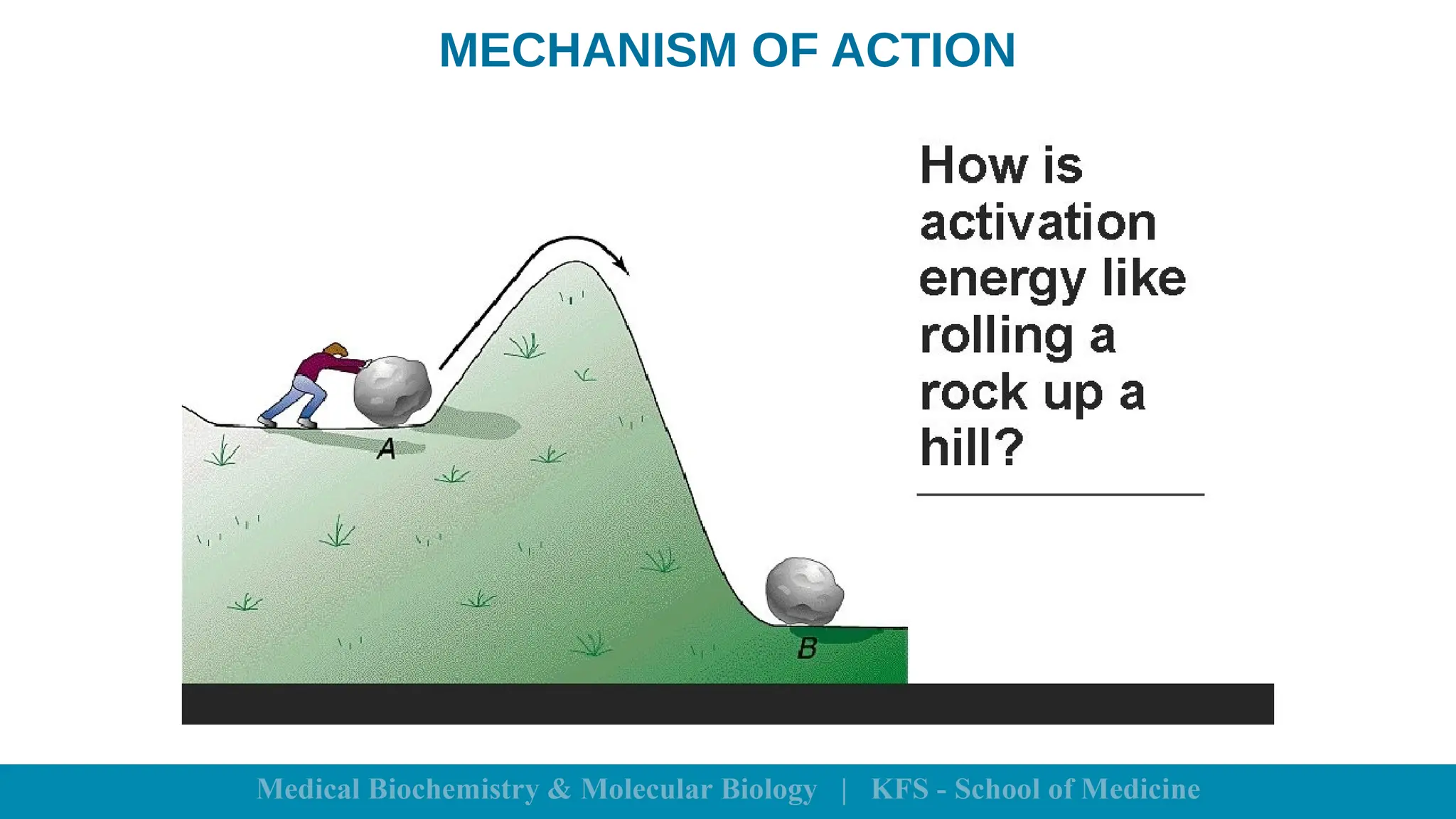
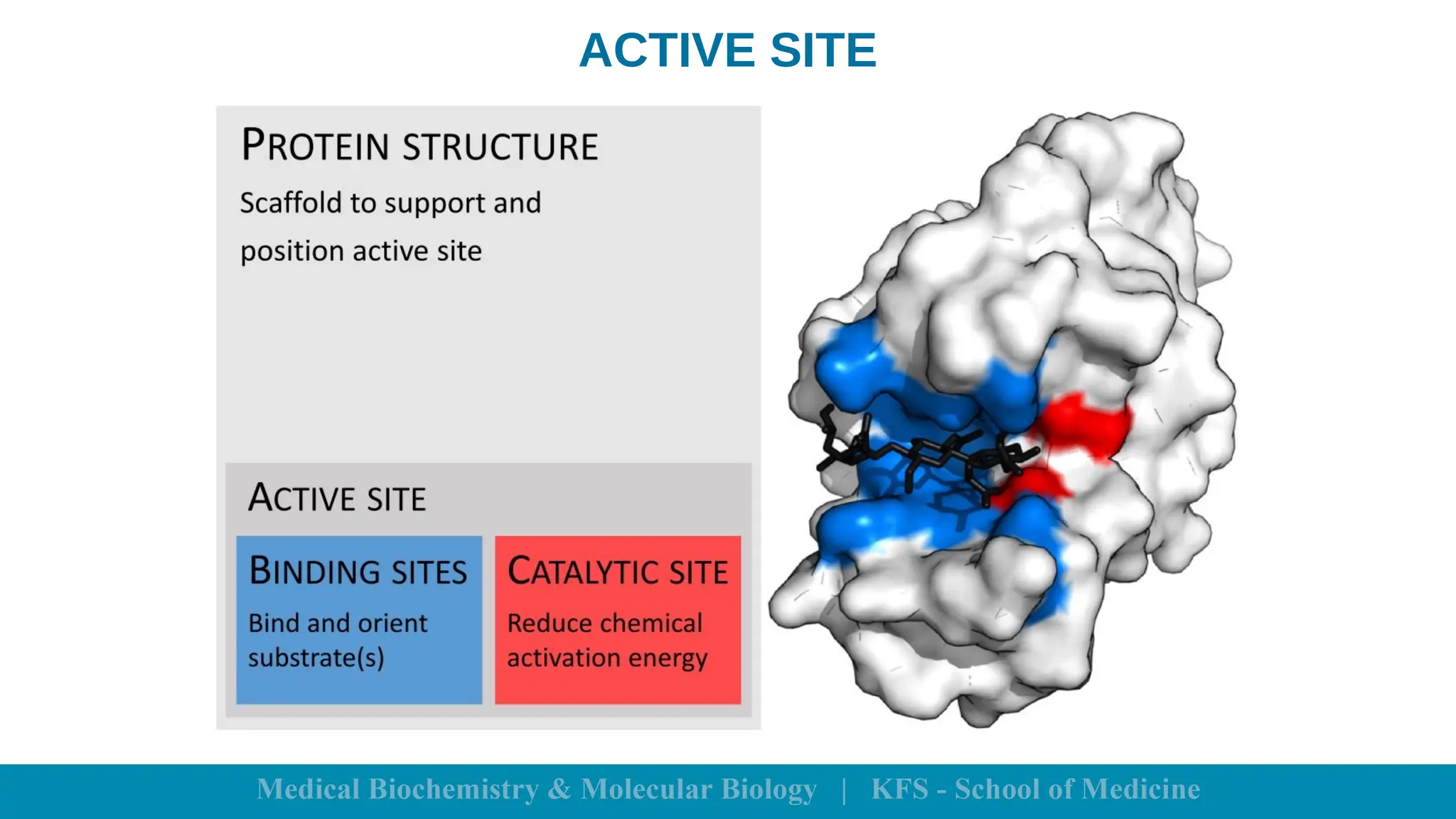
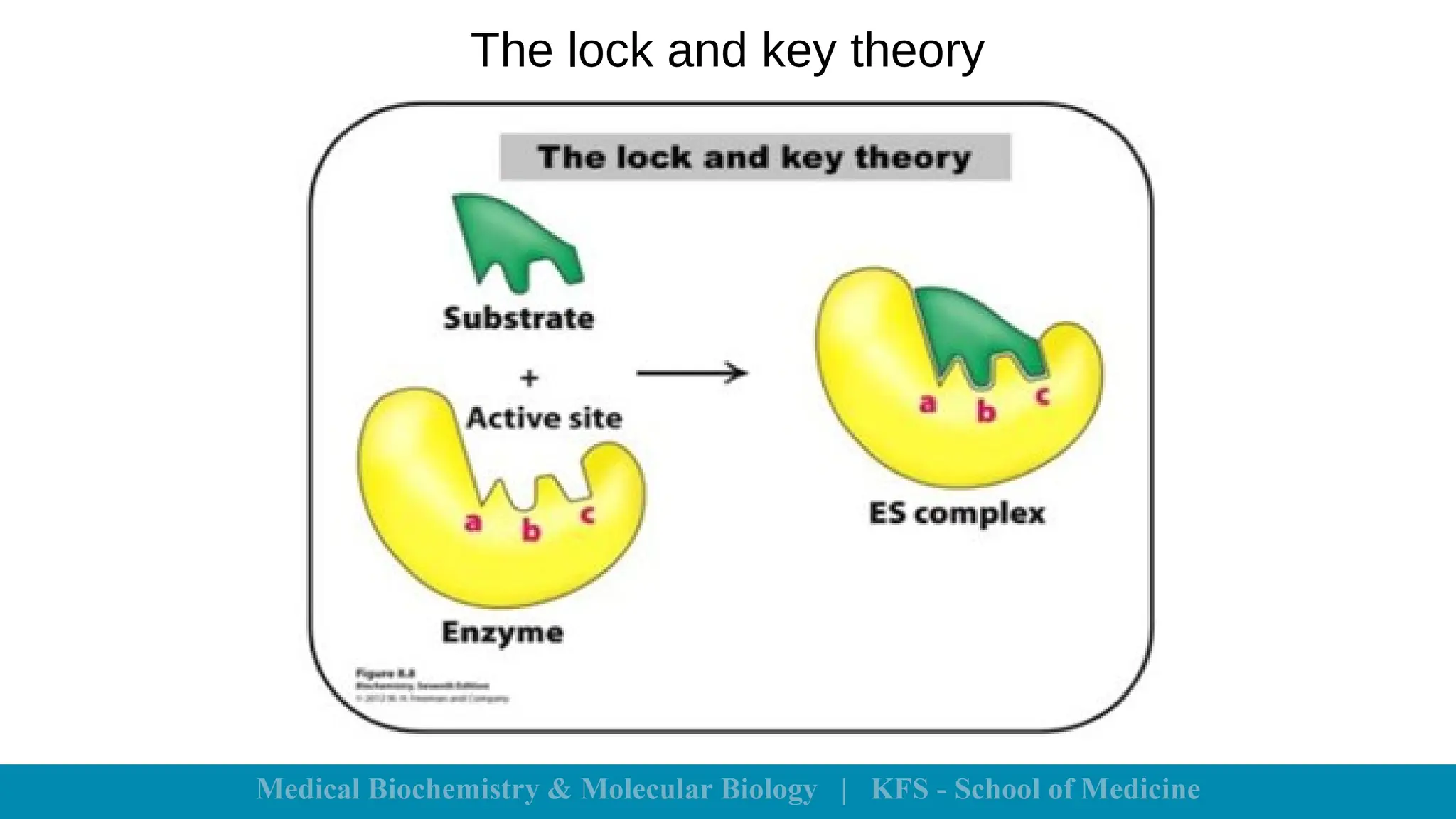
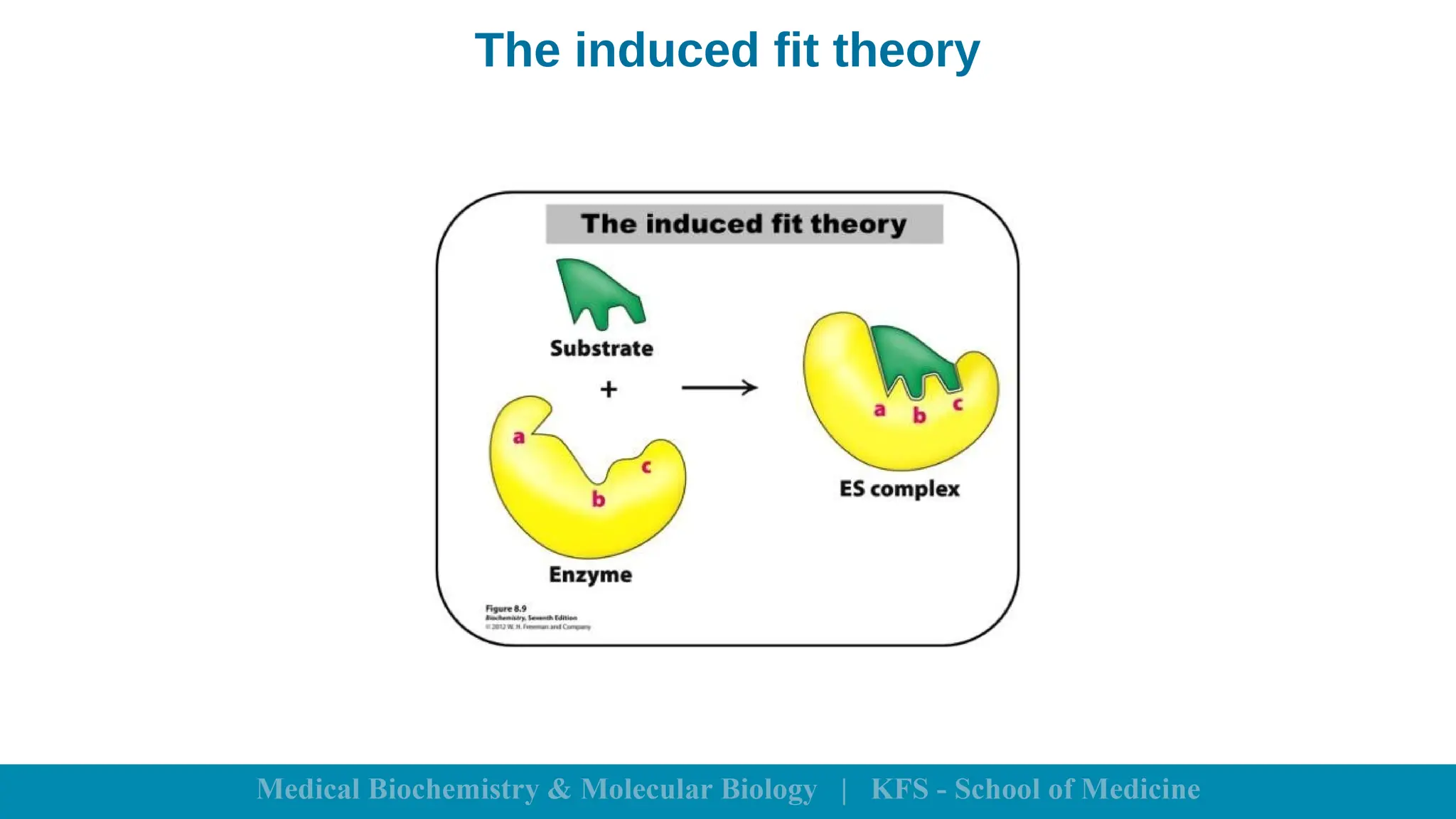
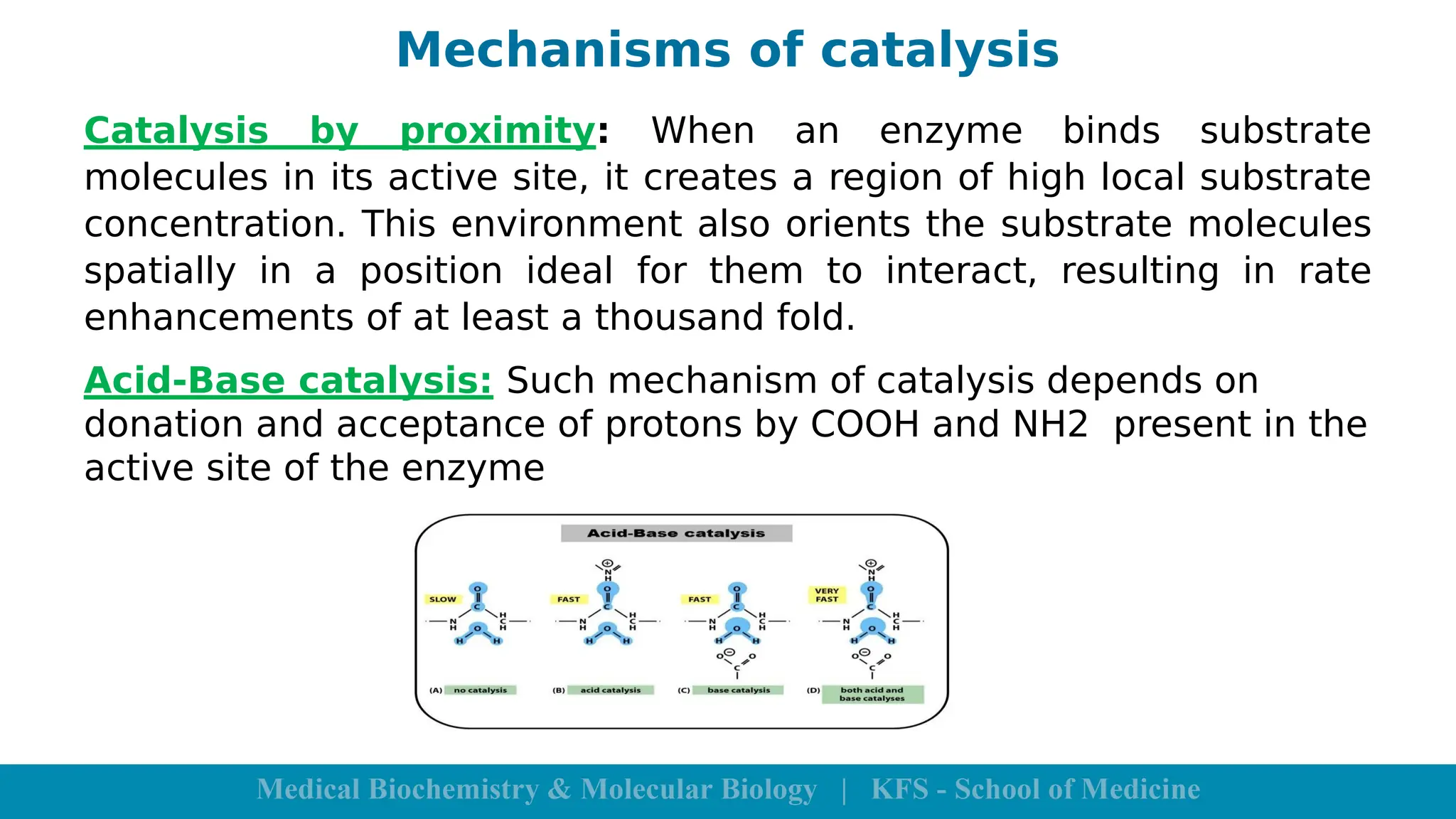
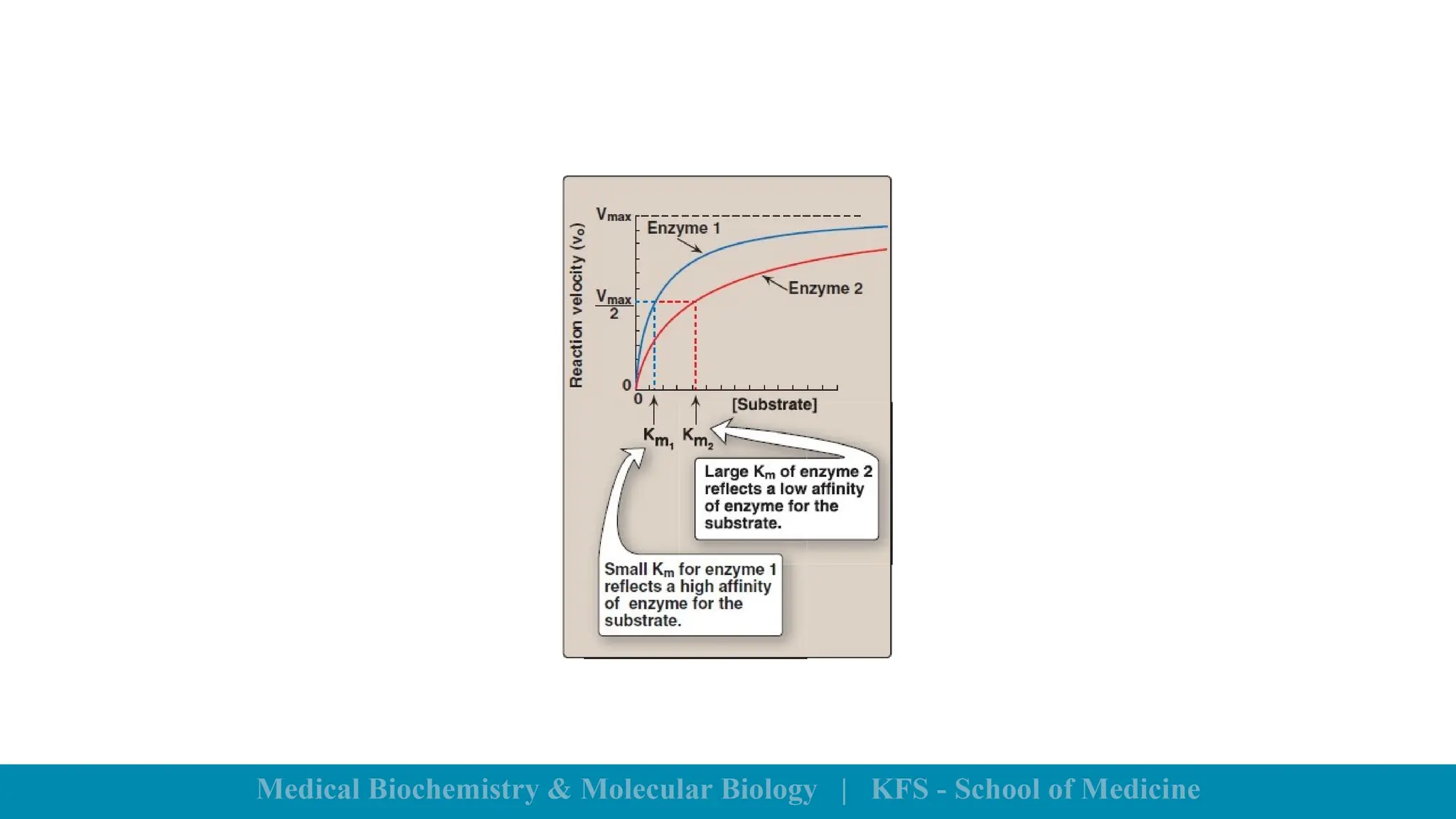
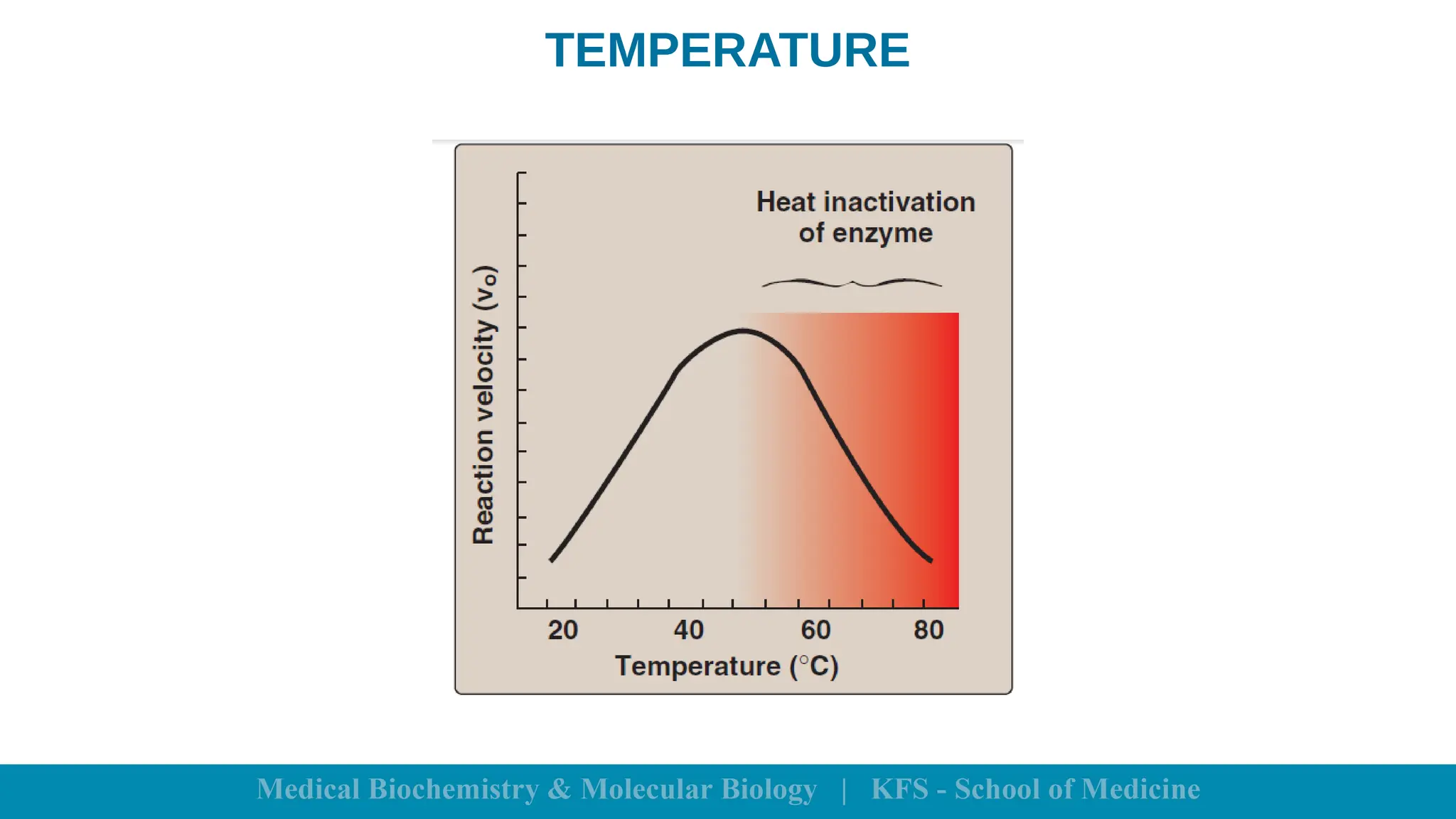
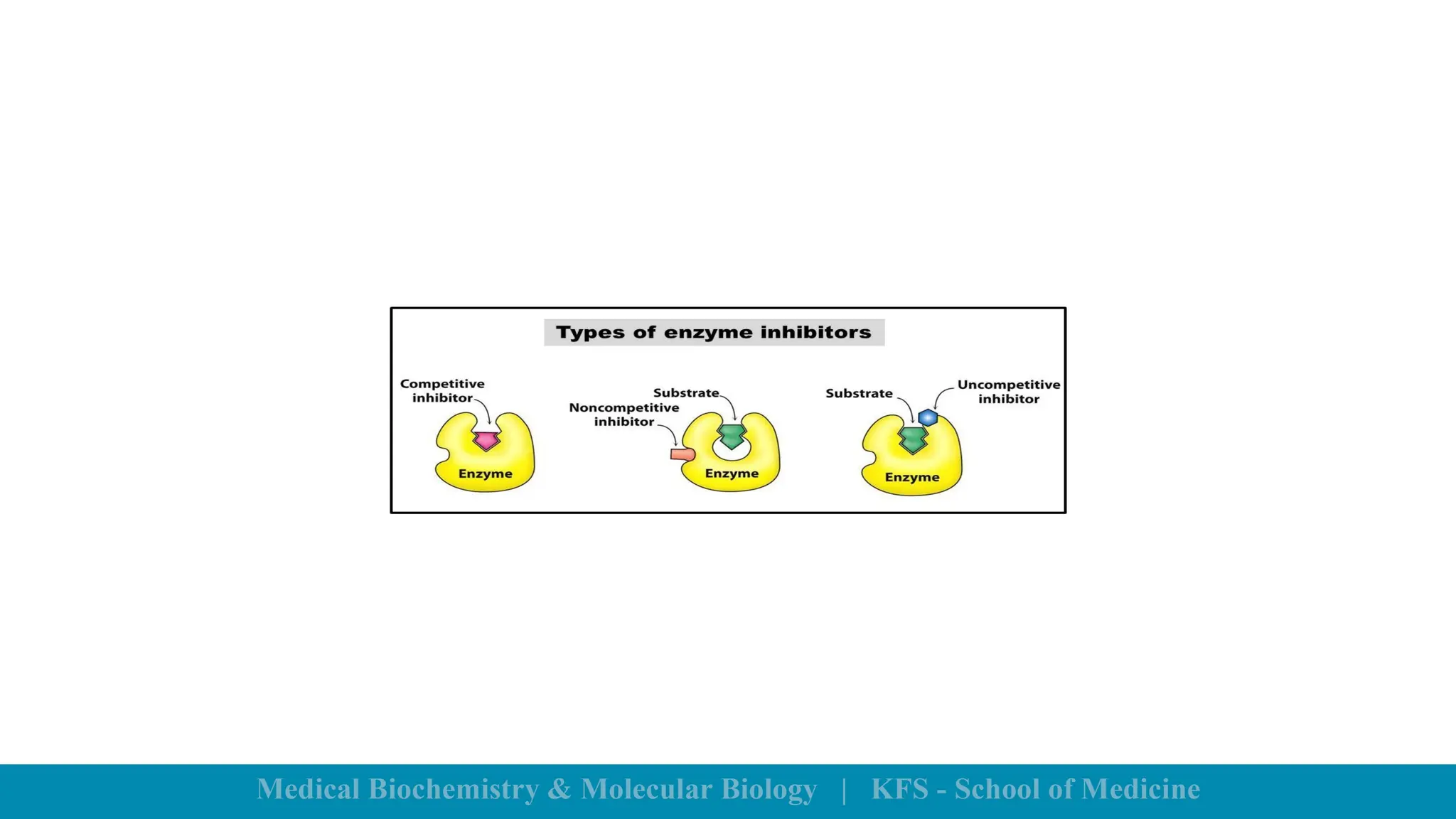
Enzymes are specific biological catalysts that accelerate chemical reactions without changing in structure or being consumed. They exhibit high specificity, various classifications, and operate optimally within particular temperature and pH ranges. Factors such as enzyme and substrate concentrations, temperature, pH, and the presence of activators or inhibitors significantly influence enzyme activity.