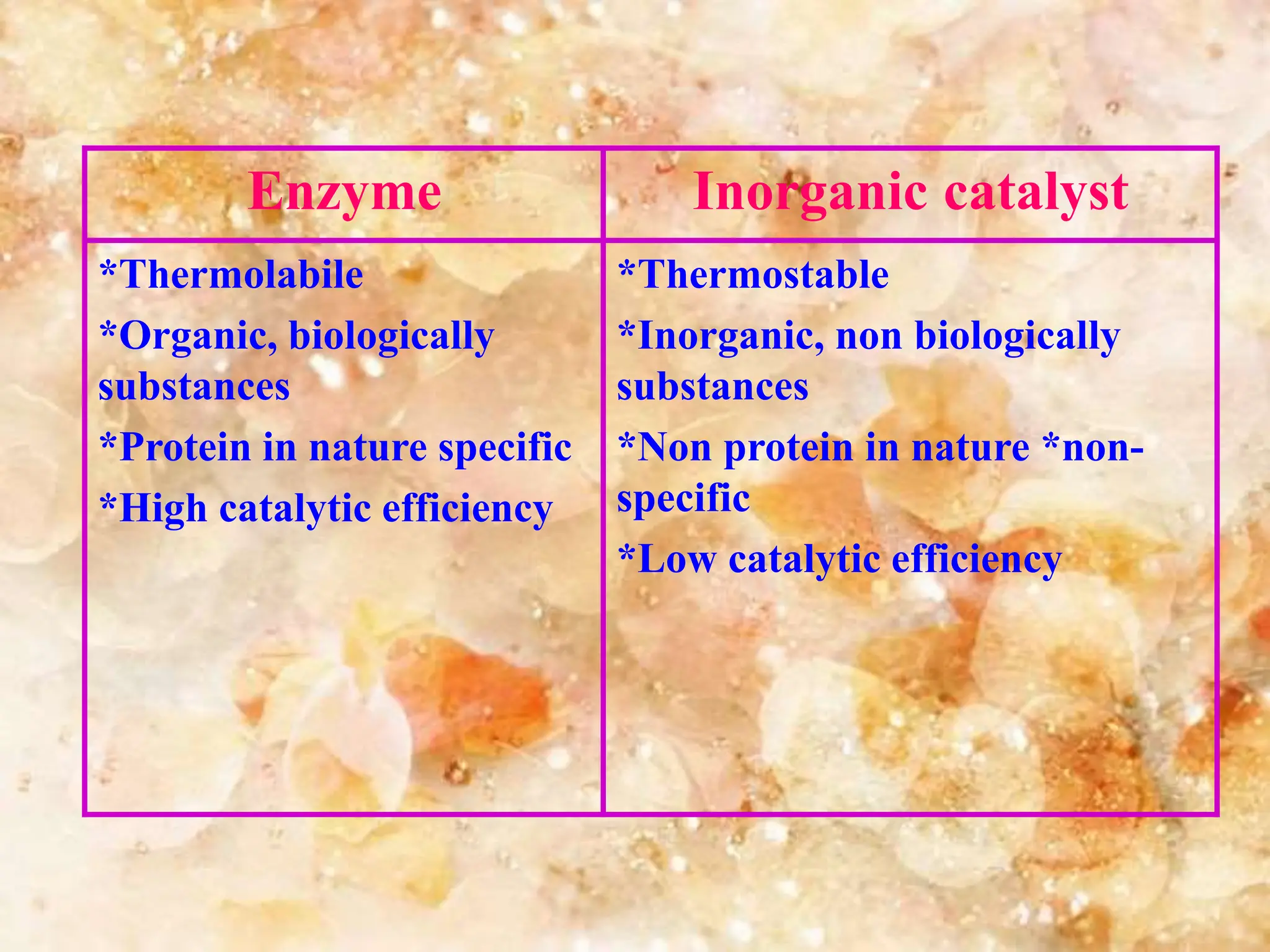
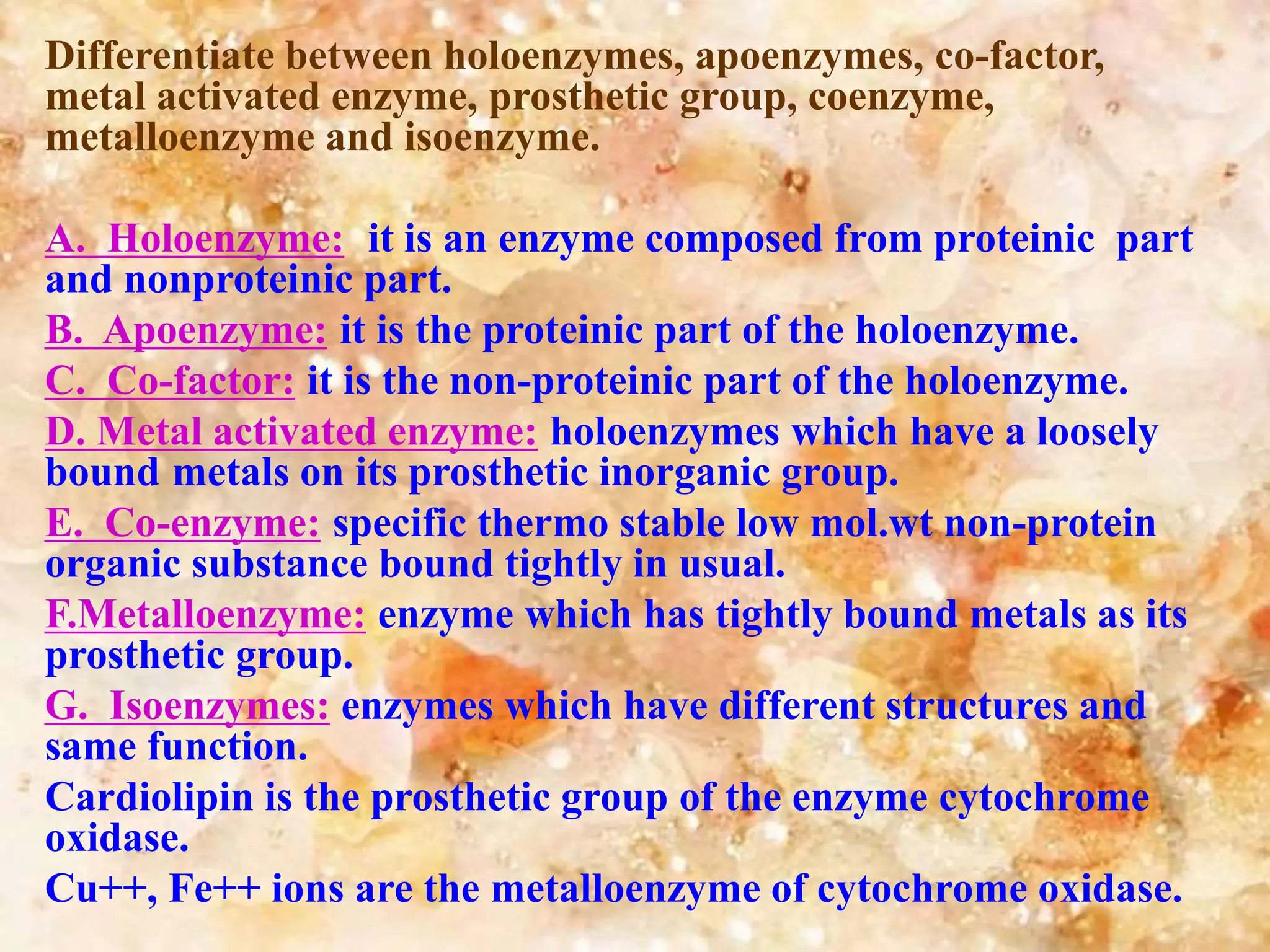
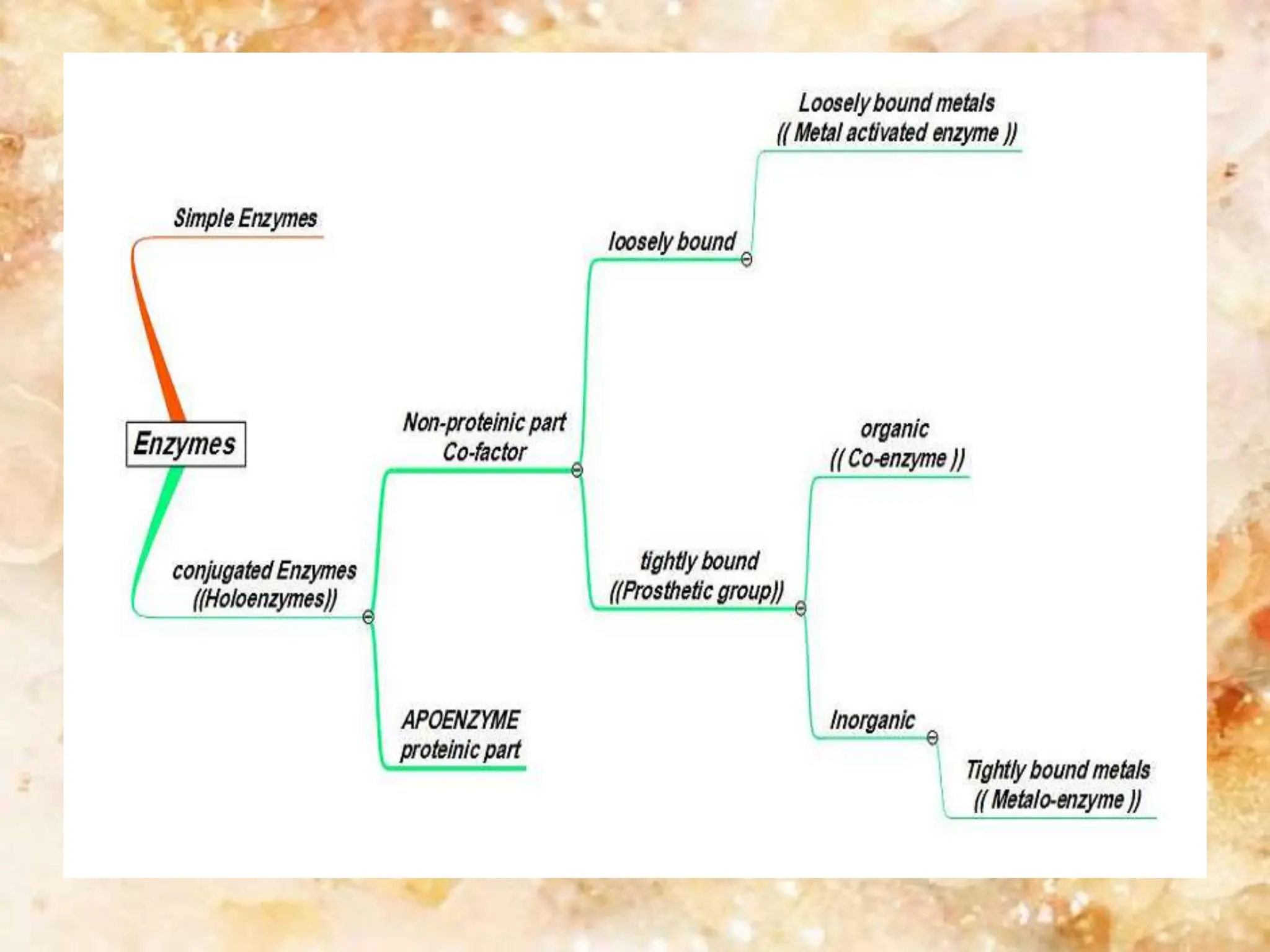
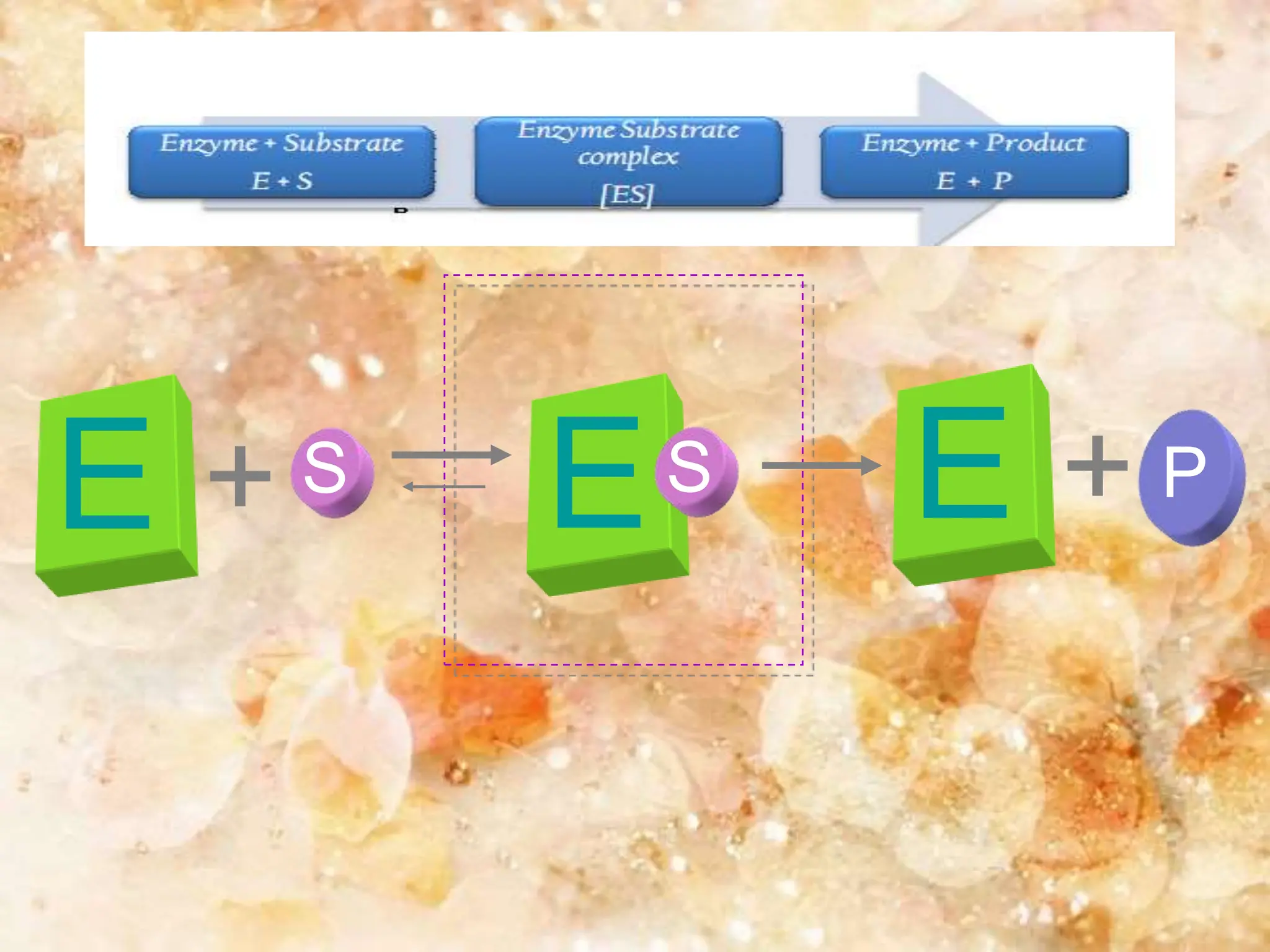
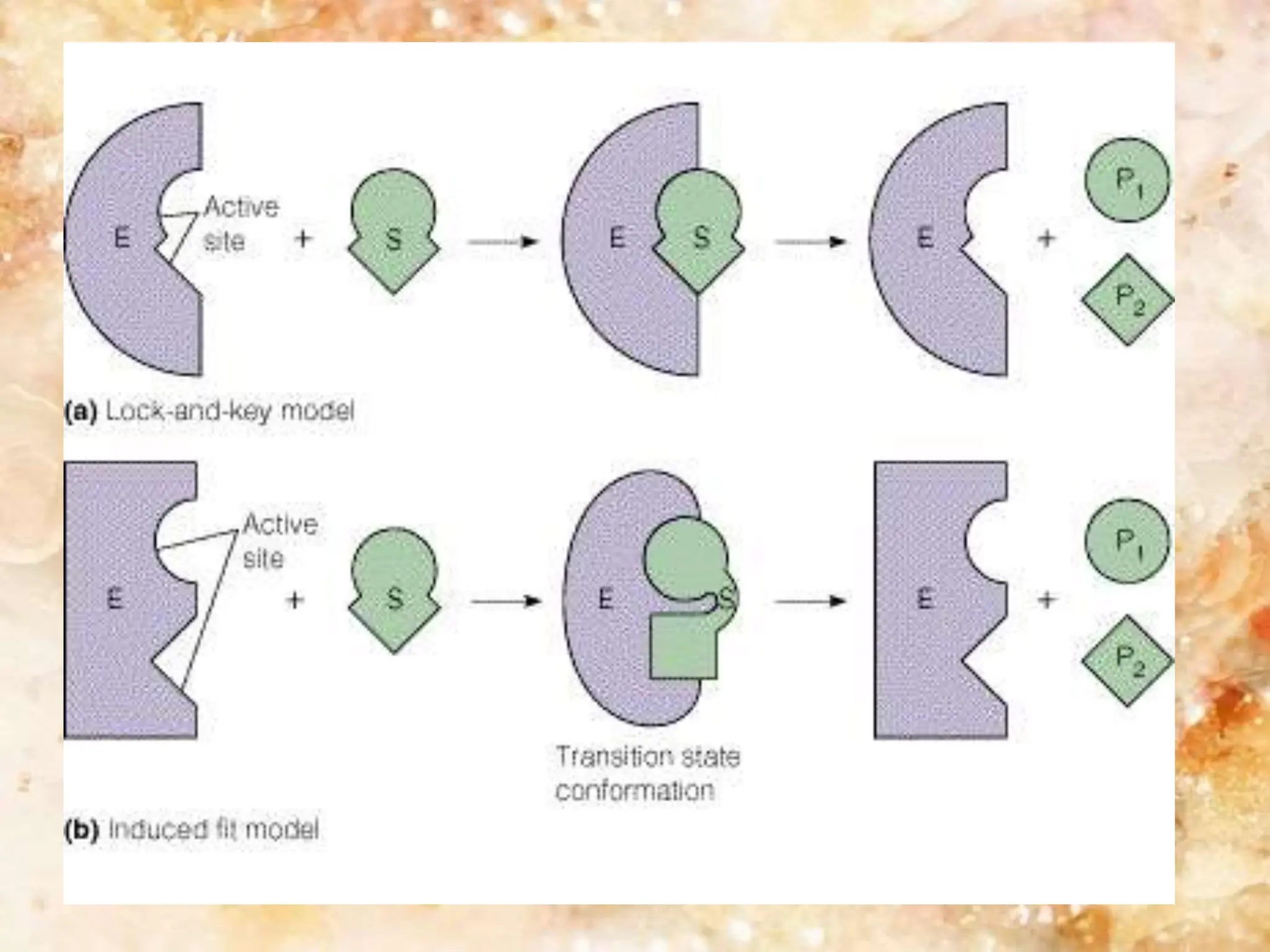
Enzymes are protein catalysts that accelerate biochemical reactions. They are produced by living organisms and are responsible for essential reactions in microbes, plants, animals, and humans. Enzymes work efficiently and specifically, catalyzing reactions that otherwise would proceed slowly or not at all. They are regulated by factors like temperature, pH, inhibitors, and activators. Enzyme activity can be inhibited through competitive inhibition, where an inhibitor binds to the active site, or non-competitive inhibition, where an inhibitor binds elsewhere and alters the enzyme's shape.





![Some vitamins act as co-enzyme:
a.B1 thiamine : form TPP [ thiamine pyrophosphate ] co-enzyme for pyruvate
degyrogenase .
b. B2 : FAD [flavin adenine dinucleotide] , FMN [flavin mononucleotide].
c. B3 :NAD [ nicotinamide adenine dinucleotide ] , NADP nicotinamide adenine
dinucleotide phosphate ]
d. B5 pantothenic :co-enzyme A [transfer of acyl group]
e. B6 pyridoxine :form co-enzyme [pyridoxal phosphate].
f.B12 cobalamin : cabamide
g. Biotin :coenzyme in carboxylation reaction.](https://image.slidesharecdn.com/enzymes-3rd-week-240219074121-8e11168d/75/Enzymes-3rd-week-ppt-enzymology-molecular-biology-8-2048.jpg)












![Michaelis constant (Km)
*Km is equal to the substrate
concentration [S] at which the
reaction is half of its maximum
(1/2Vmax).
*It expresses the affinity of the
enzyme to its substrate.
*Low Km means high affinity of the
enzyme to the substrate
*High Km means low affinity of the
enzyme to the substrate](https://image.slidesharecdn.com/enzymes-3rd-week-240219074121-8e11168d/75/Enzymes-3rd-week-ppt-enzymology-molecular-biology-24-2048.jpg)















![Enzyme Inhibition (Mechanism)
S I
I
I
I
S
Competitive Non-competitive
E
Different site
Compete for
active site
Inhibitor
Substrate
[I] binds to free [E] only,
and competes with [S];
increasing [S] overcomes
Inhibition by [I].
[I] binds to free [E] or [ES]
complex; Increasing [S] can
not overcome [I] inhibition.
E + S→ES→E + P
+
I
↓
EI
←
↑
E + S→ES→E + P
+ +
I I
↓ ↓
EI+S→EIS
←
↑ ↑
E
I
S](https://image.slidesharecdn.com/enzymes-3rd-week-240219074121-8e11168d/75/Enzymes-3rd-week-ppt-enzymology-molecular-biology-40-2048.jpg)




































