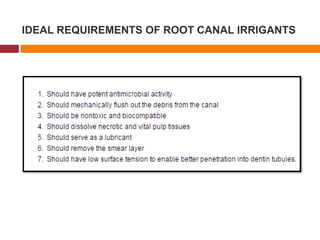
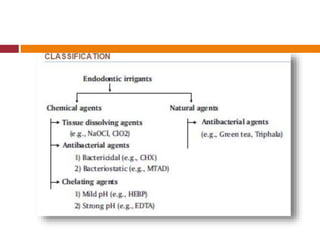
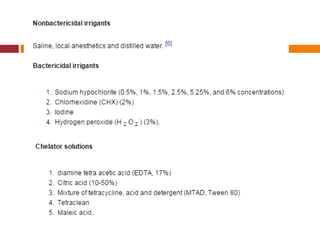
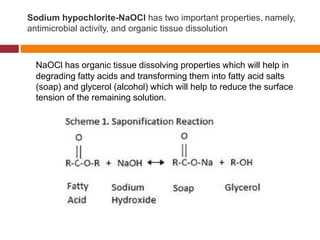
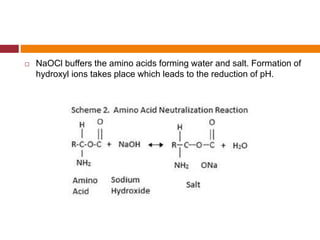
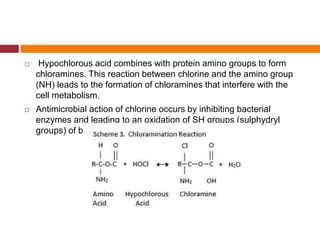
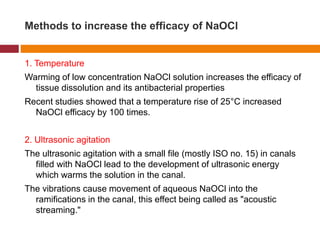
This document summarizes various endodontic irrigants used during root canal treatment. It describes the properties and effectiveness of sodium hypochlorite (NaOCl), chlorhexidine (CHX), iodine, ethylenediaminetetraacetic acid (EDTA), citric acid, a mixture of tetracycline, acid and detergent (MTAD), Tetraclean, maleic acid, bis-dequalinium acetate (BDA), triclosan with Gantrez, and chlorine dioxide. It provides details on how each irrigant aids in disinfection, removal of smear layer and debris, and substantivity within the root canal system. The document emphasizes