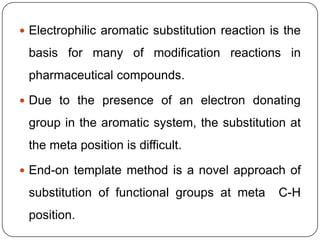
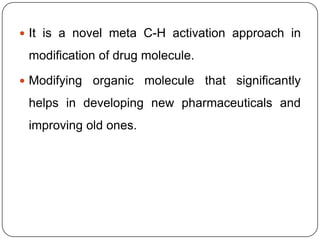
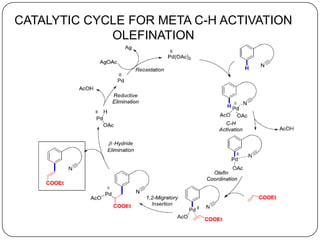
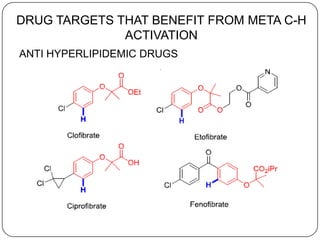
This document discusses an end-on template method for meta C-H activation in pharmaceutical chemistry. The method uses a temporary helper molecule or template to mediate the addition of functional groups to carbon atoms. A metal catalyst like palladium helps facilitate the process. The template approach allows for easier modification of drug molecules by attaching biologically active functional groups at meta positions. Several drug targets that could benefit from this meta C-H activation are discussed, including antihyperlipidemic, GABA agonist, anti-cancer and antihypertensive drugs.