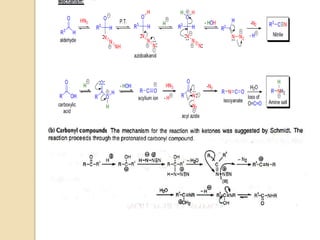
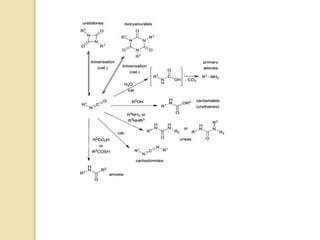
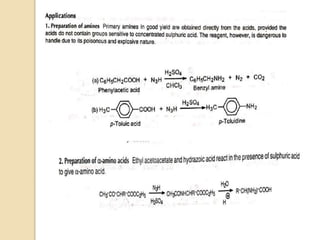
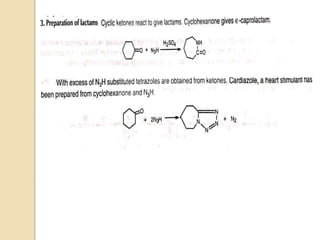
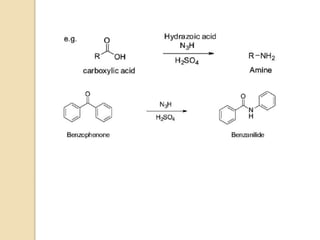
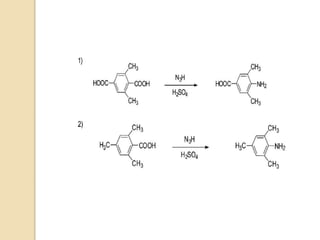
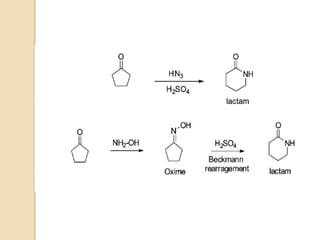
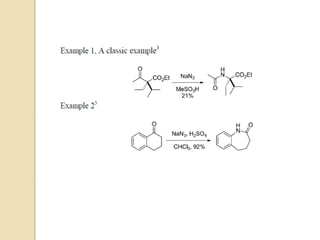
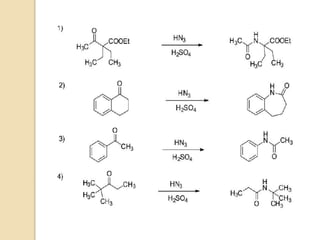
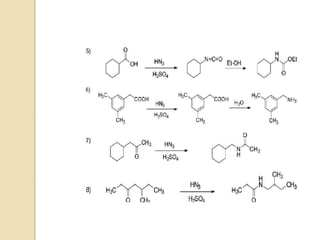
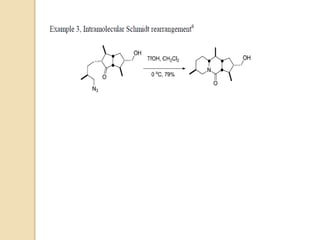
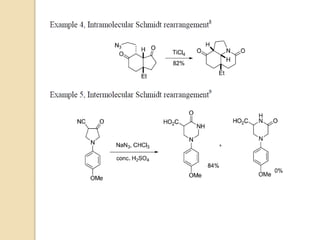
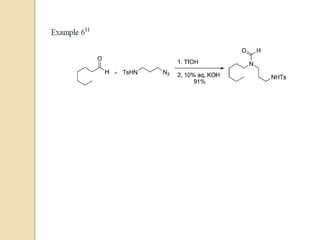
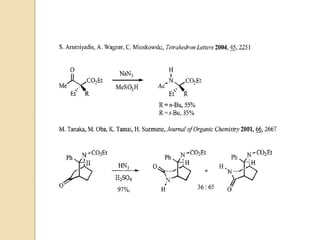
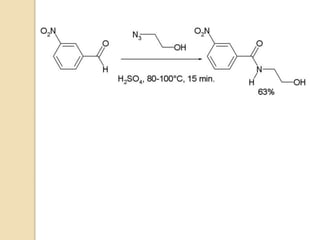
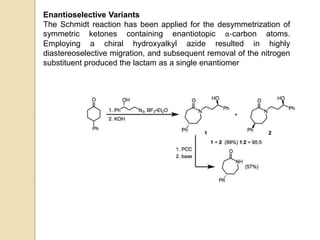
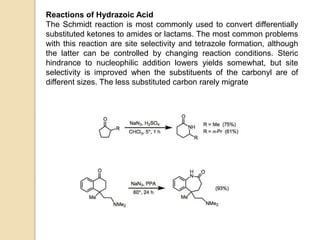
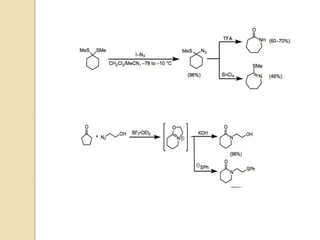
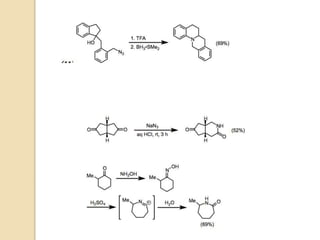
The Schmidt reaction involves reacting an azide with a carbonyl compound like an aldehyde, ketone, or carboxylic acid under acidic conditions. This results in the formation of an amine or amide with the expulsion of nitrogen. The reaction was first reported in 1924 by Karl Friedrich Schmidt and involves the migration of a carbonyl substituent to the nitrogen atom of the azide. The Schmidt reaction is useful for synthesizing amines, amides, nitriles, and lactams and has been applied to natural product synthesis. It can proceed intramolecularly and variants have been developed to achieve enantioselectivity.