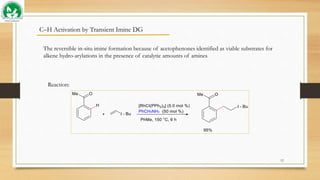
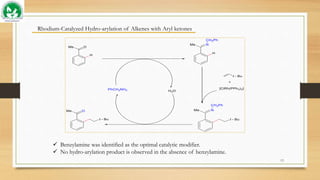
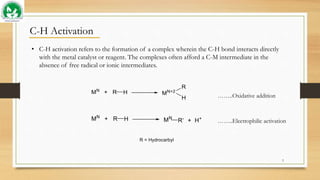
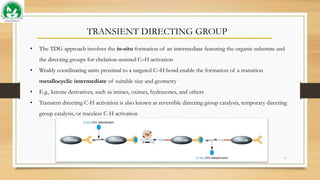
The document discusses transient ligand-based C-H activation at sp2 centers. It describes how transient directing groups (TDGs) can be used to overcome limitations of conventional directing group approaches. TDGs involve the in-situ formation of coordinating groups near targeted C-H bonds to enable regioselective metallocyclic intermediates. Examples are given of using transient phosphite and imine groups formed from substrates like phenols and ketones to enable C-H functionalization through reversible coordination and reductive elimination. The approach reduces steps compared to traditional cross-couplings through temporary and traceless activation of inert C-H bonds.






![10
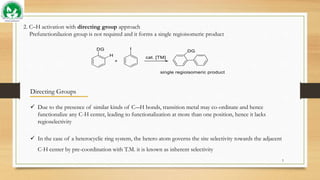
C–H Activation by Transient Phosphite DG
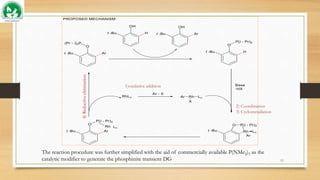
The catalytic cycle was proposed to proceed by
1. oxidative addition of aryl bromides to rhodium(I),
2. coordination of the phosphinite co-catalyst to rhodium(III) species, and
3. subsequent cyclometallation followed by
4. reductive elimination to deliver the ortho-arylated phosphinite,
which generates the co-catalyst, and liberates the 2-arylated product by transesterification
ortho-C–H arylation of phenols with an aryl halide
[RhCl(PPh3)3] - Wilkinson’s catalyst](https://image.slidesharecdn.com/g11ppt-220517103851-b42fc7c2/85/Transient-ligand-based-C-H-activation-10-320.jpg)