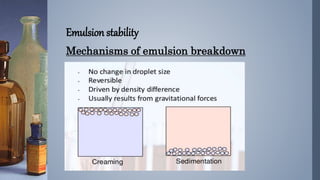
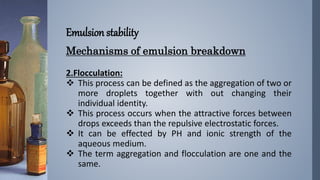
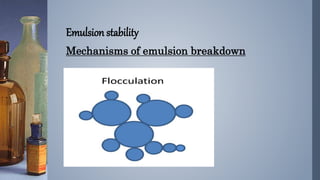
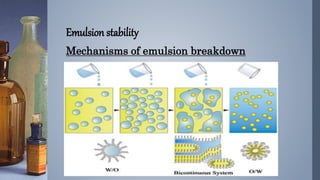
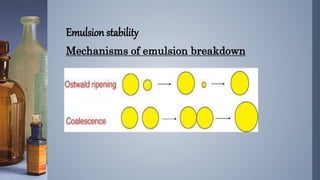
Emulsions are thermodynamically unstable and tend to separate over time through various mechanisms such as creaming, sedimentation, flocculation, coalescence, phase inversion, and Ostwald ripening. Emulsion stability is conferred by emulsifying agents which can stabilize emulsions through three theorized mechanisms: forming a protective interfacial film, reducing interfacial tension between the dispersed and continuous phases, or orienting at the interface in a way that depends on their hydrophilic/hydrophobic properties.