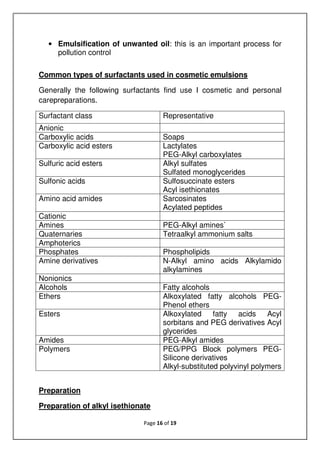
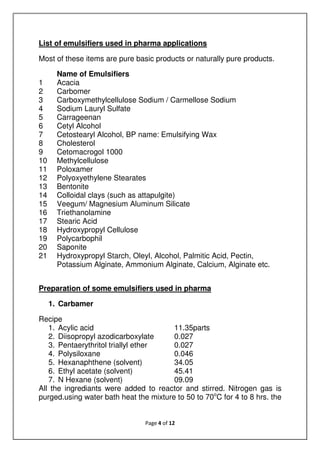
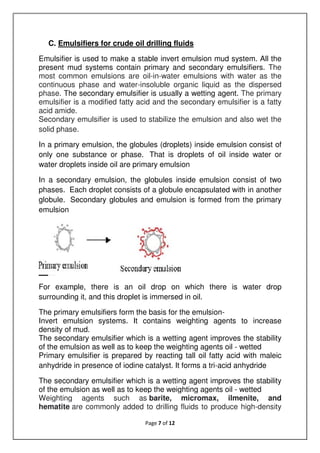
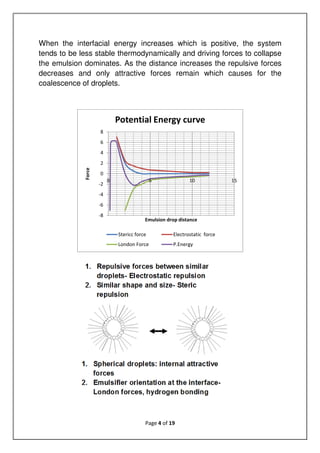
The document discusses emulsions, which are mixtures of two immiscible liquids, and the role of emulsifiers in stabilizing them. It explains the science behind emulsions, including their thermodynamic instability, Brownian motion, and the types of emulsifiers used based on their hydrophilic-lipophilic balance (HLB). The document also details the mechanisms of emulsion breakdown, such as creaming and coalescence, and various methods for calculating the effectiveness of surfactants.










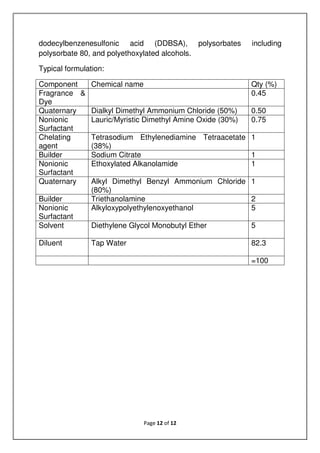
![Page 13 of 19
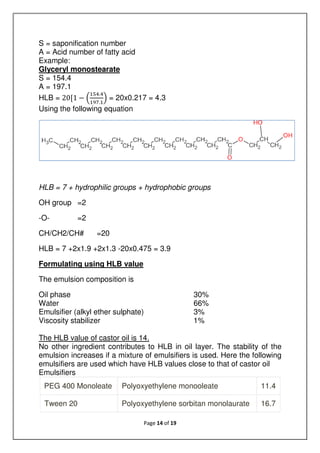
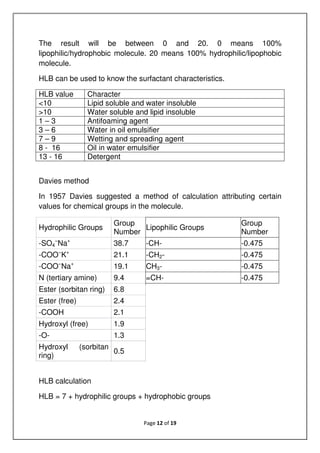
Example: PEG400 monooleate
Group Value No Total
Free OH 1.9 1 +1.9
-O- 1.3 8 +10.4
CH/CH2/CH3 -0.475 17 -8.075
HLB = 7+1.9+10.4-8.075 = 11.2
HLB is not considering, pH, temperature influence, emulsifier
concentration, molrcular interaction and concentration of both phases.
Emulsion stabilization using HLB value
To make a stable emulsion, the emulsifier or emulsifier mixture used
should have HLB value equal to the HLB value of the oil phase.
For nonionic surfactants, with polyoxyethylene as hydrophilic part the
HLB can be calculated using the formula
HLB=
E= percentage weight of ethylene oxide
Example: Nonylphenol 10 ethoxylate
Mol wt = 660
% EO = = = 66.67
HLB =
.
= 13.3
The HLB of Esters of polyhydric alcohols can be determined using the
following equation
HLB = 20[1 − ]](https://image.slidesharecdn.com/emulsionandemulsifiers-230308164533-c0c40255/85/Emulsion-and-Emulsifiers-pdf-13-320.jpg)