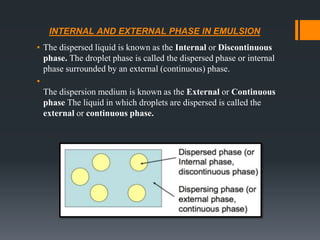
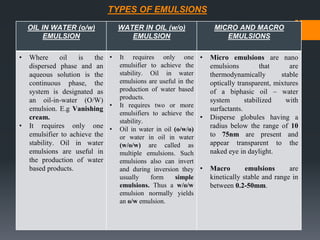
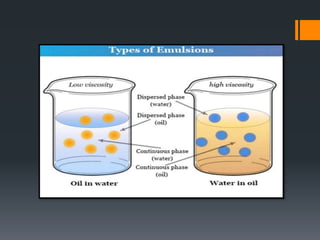
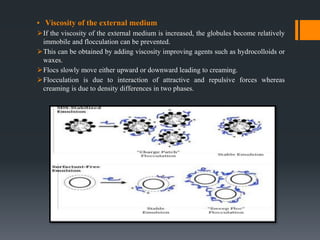
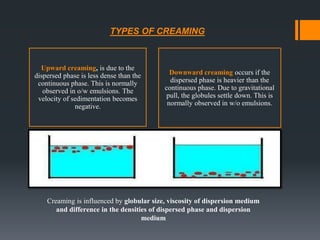
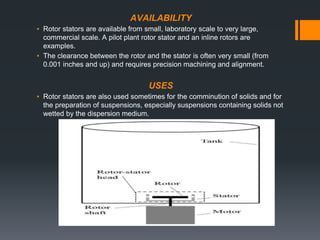
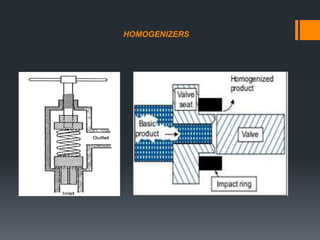
This document discusses emulsions, including their definition, types, formulation considerations, and stability factors. It defines emulsions as dispersions of one liquid in another immiscible liquid, stabilized by an emulsifying agent. The key types described are oil-in-water and water-in-oil emulsions. Important formulation considerations include phase ratio, droplet size, viscosity, density differences, and use of emulsifiers. Factors affecting stability include creaming, flocculation, coalescence, phase inversion, and Ostwald ripening. Methods to enhance stability include reducing particle size and density differences and increasing viscosity.