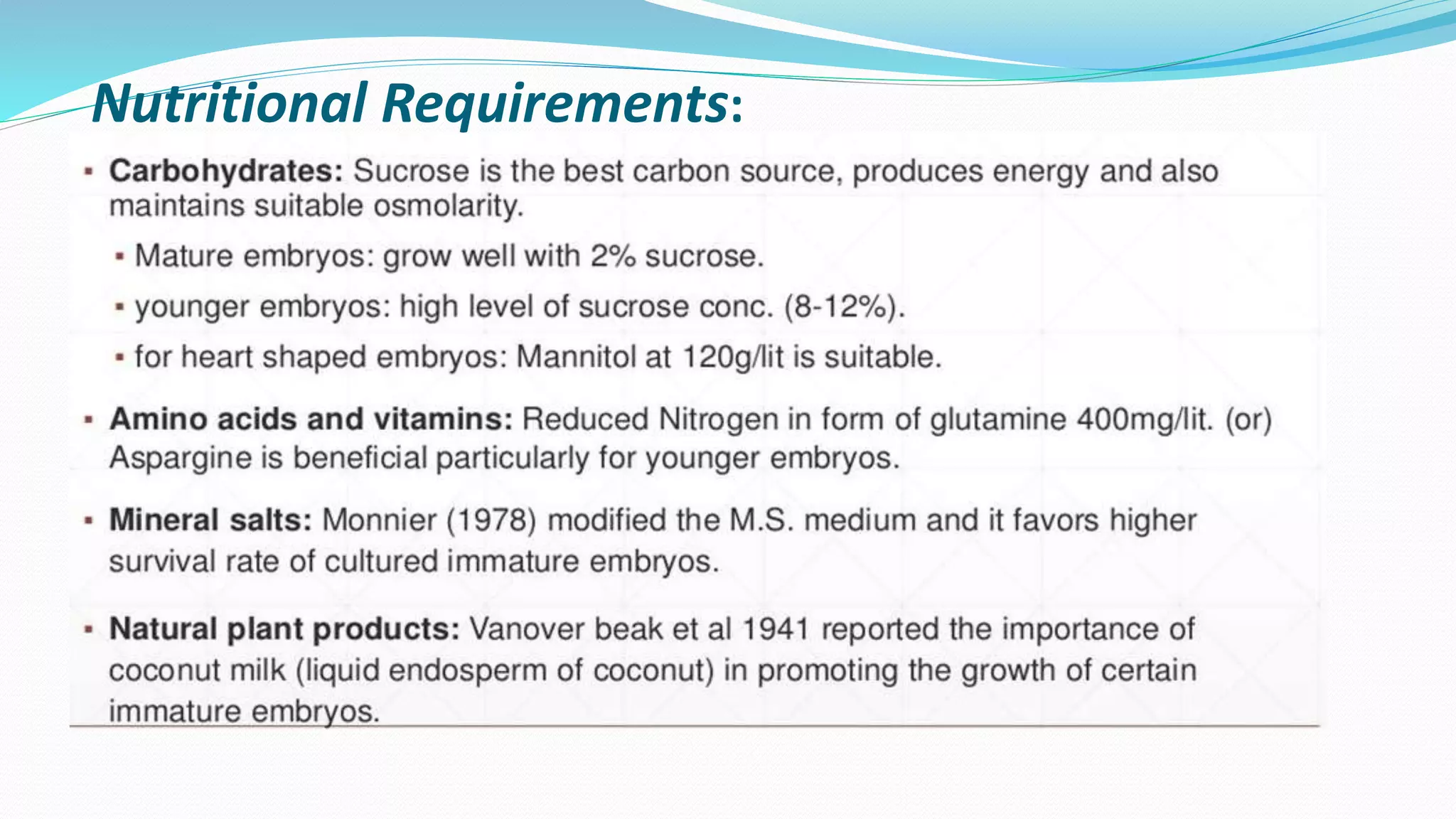
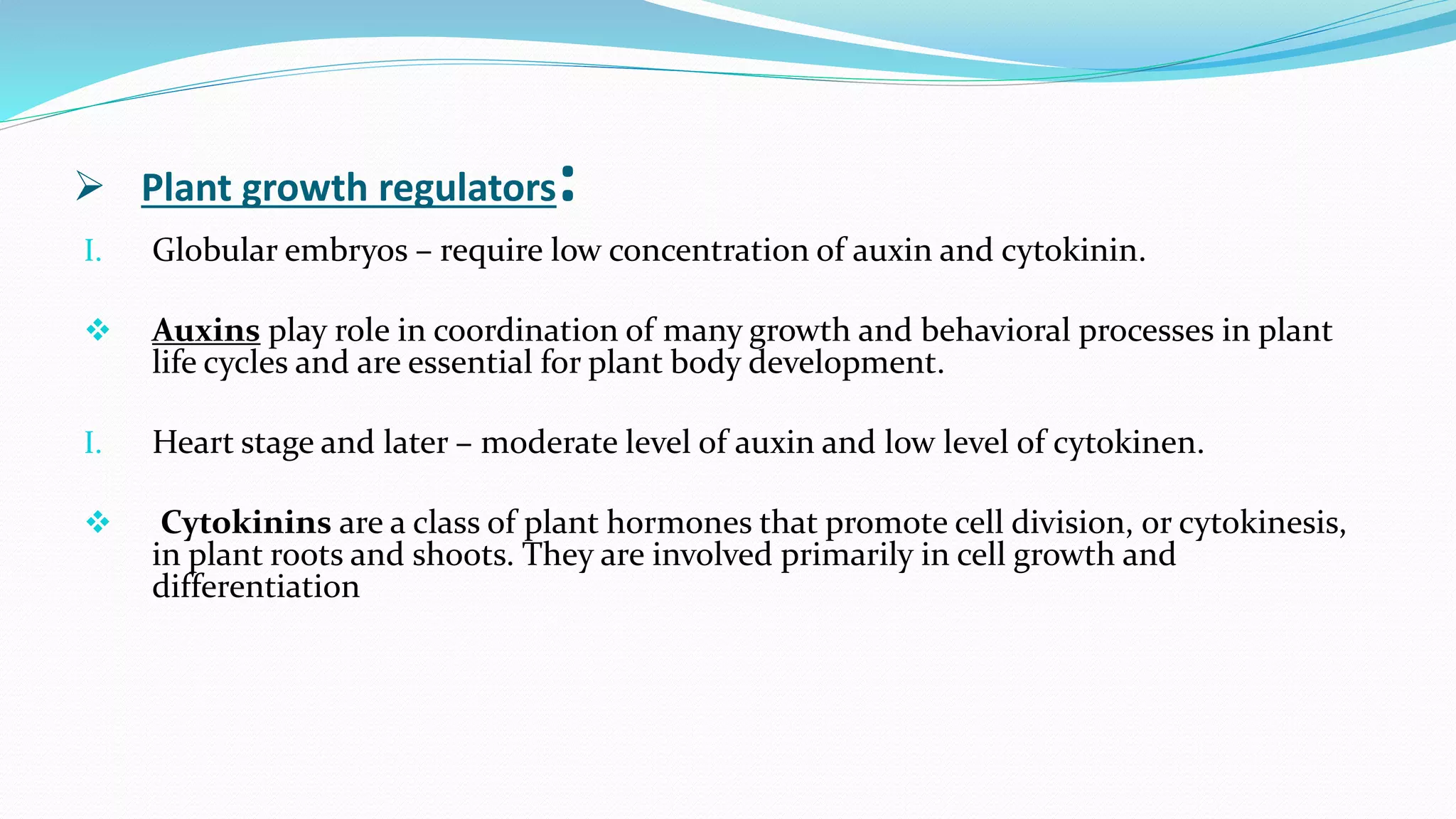
The document discusses embryo culture, which involves excising and growing embryos under artificial conditions to produce viable plants, including techniques for both mature and immature embryos. It highlights the importance of cultural conditions, media composition, and specific protocols for plants like maize and other species, detailing steps for overcoming seed dormancy and embryo abortion. Applications of embryo culture encompass producing haploid plants and rescuing embryos from wide crosses.