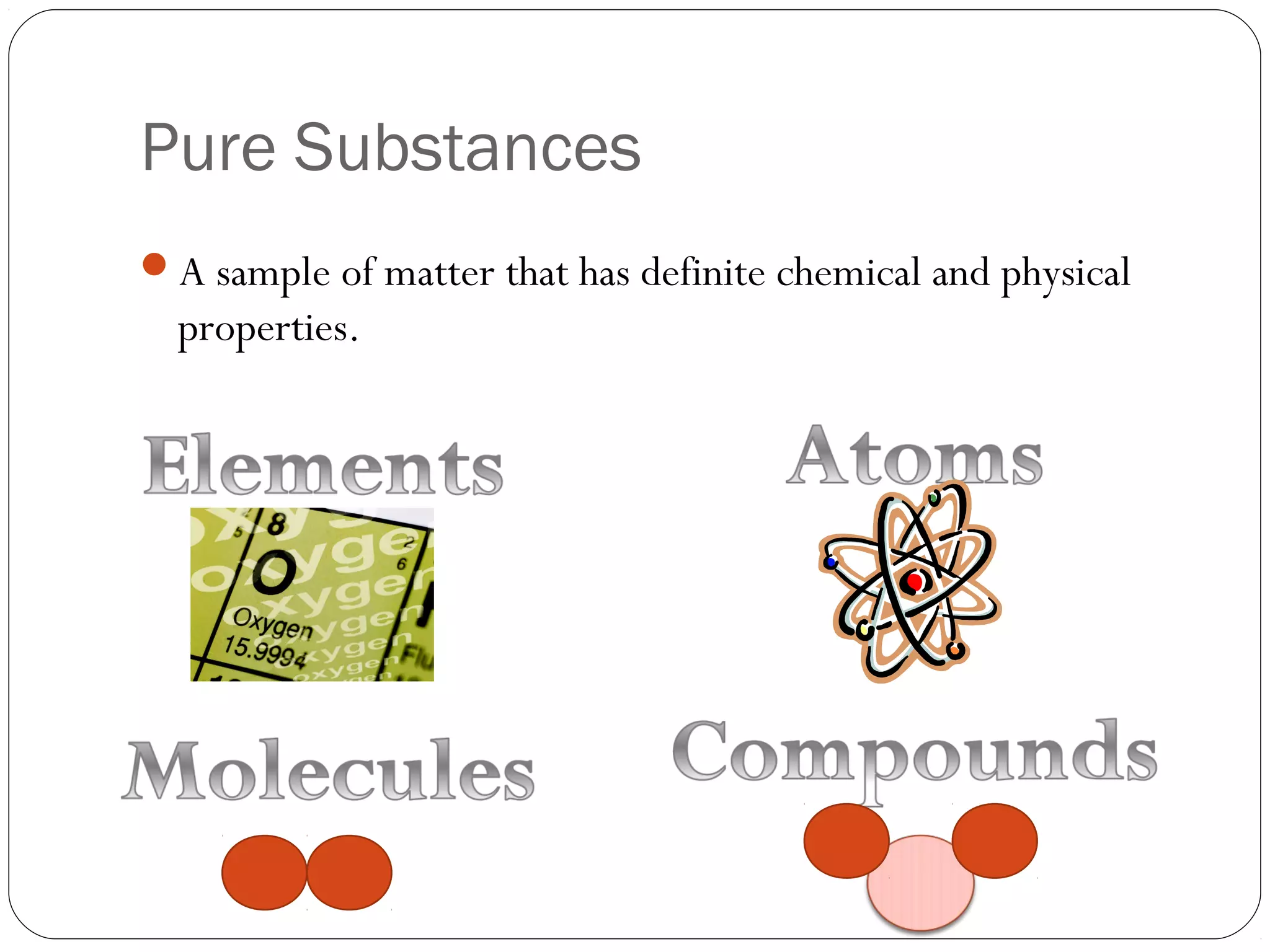
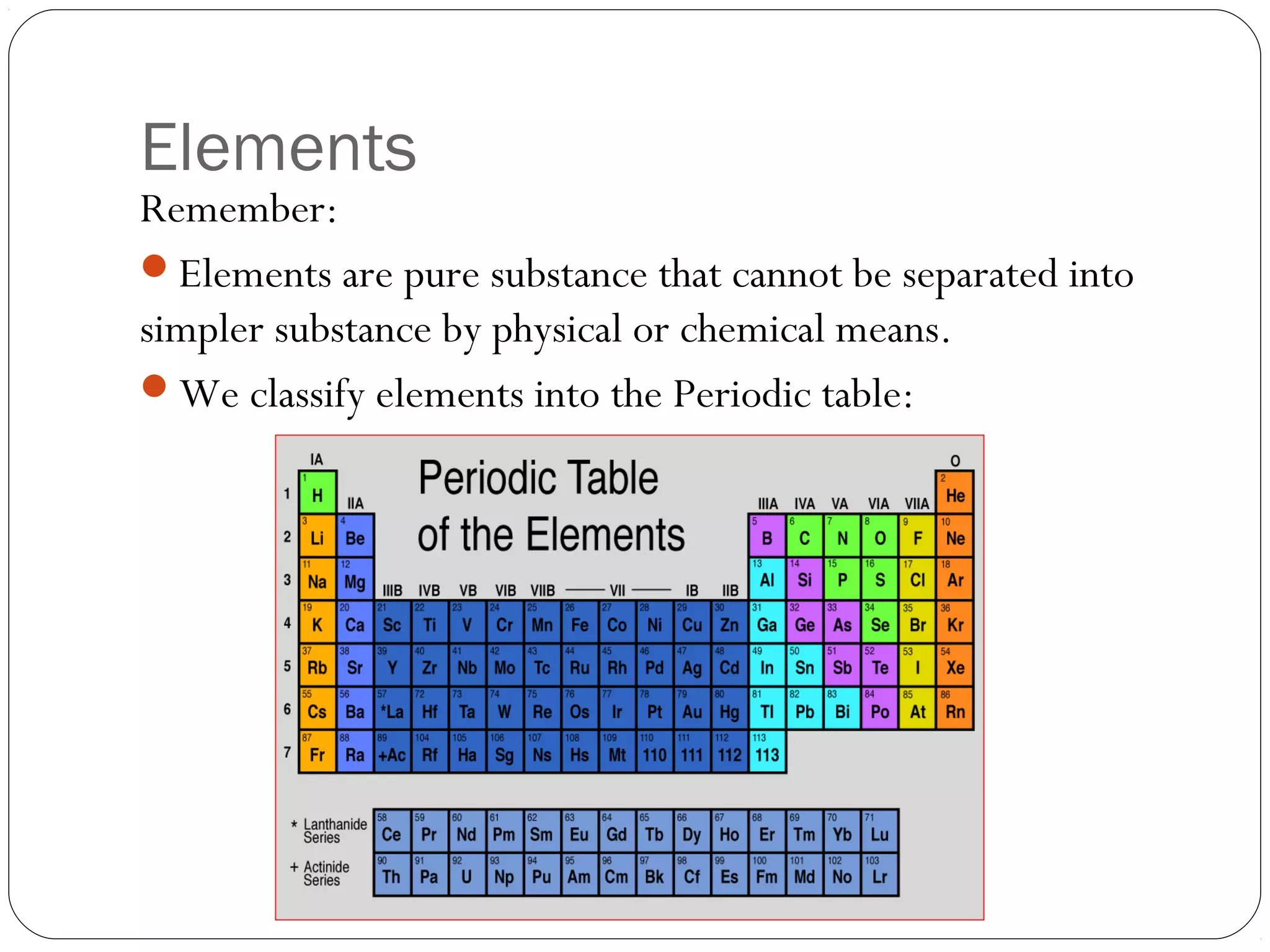
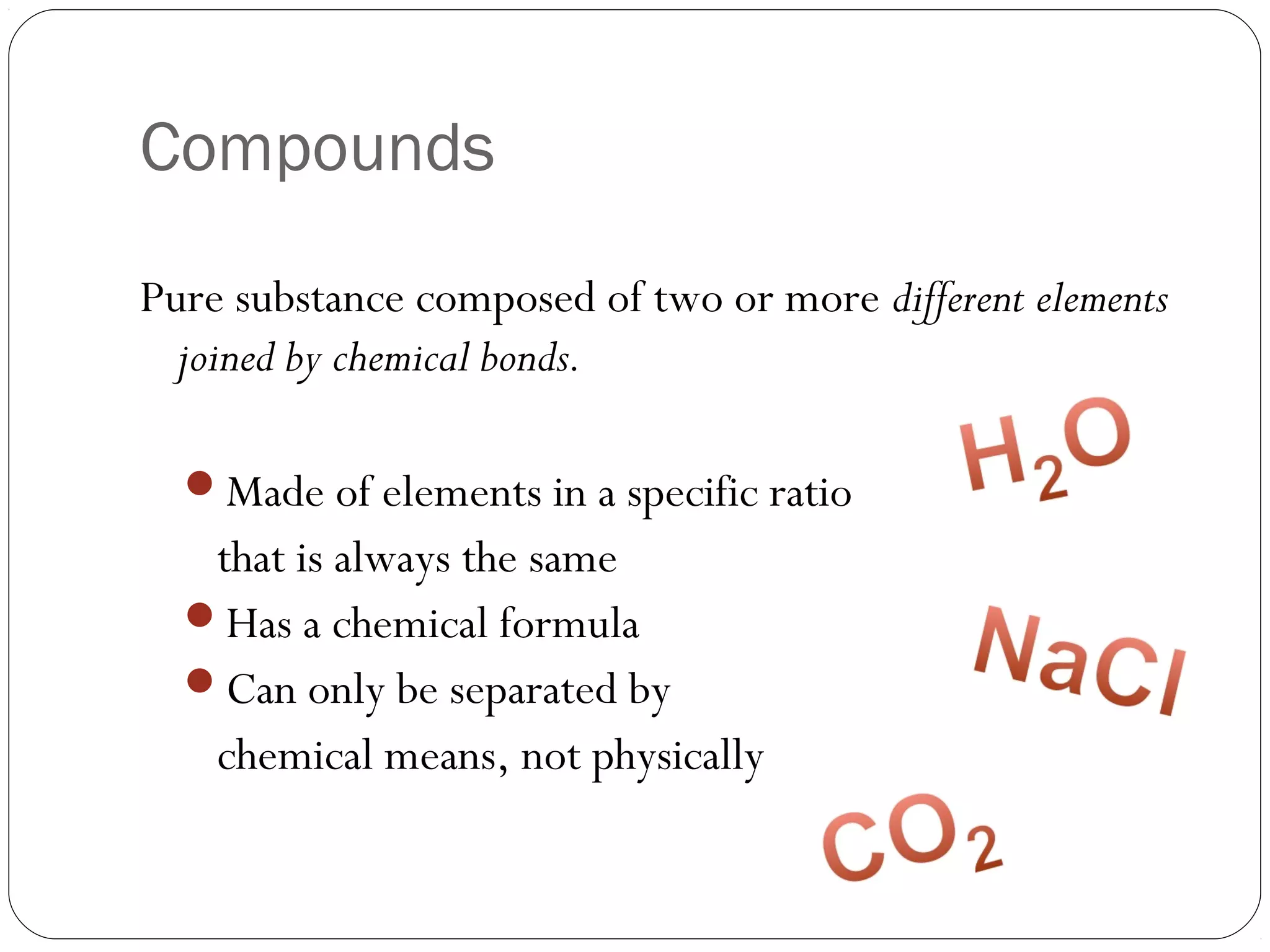
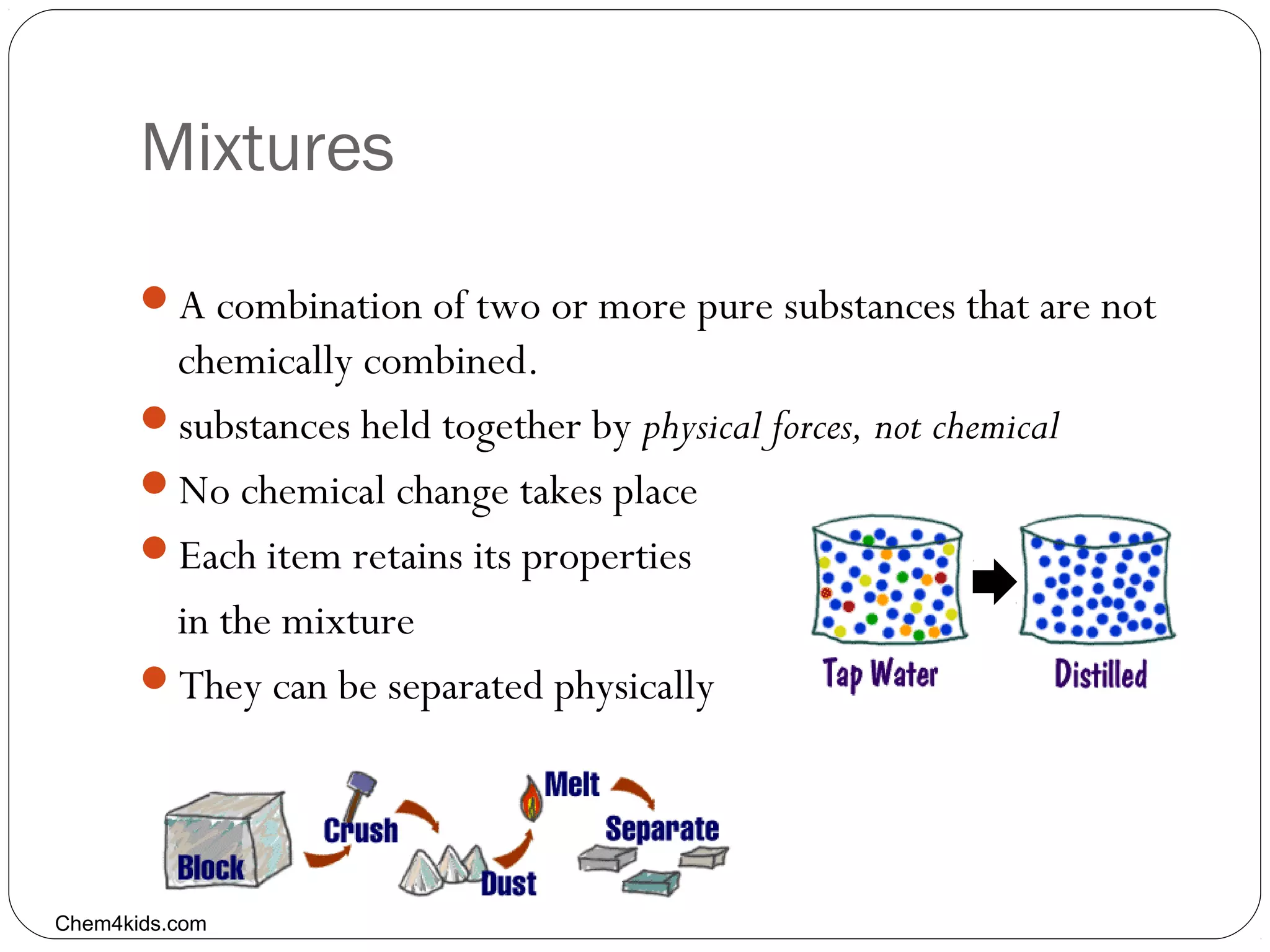
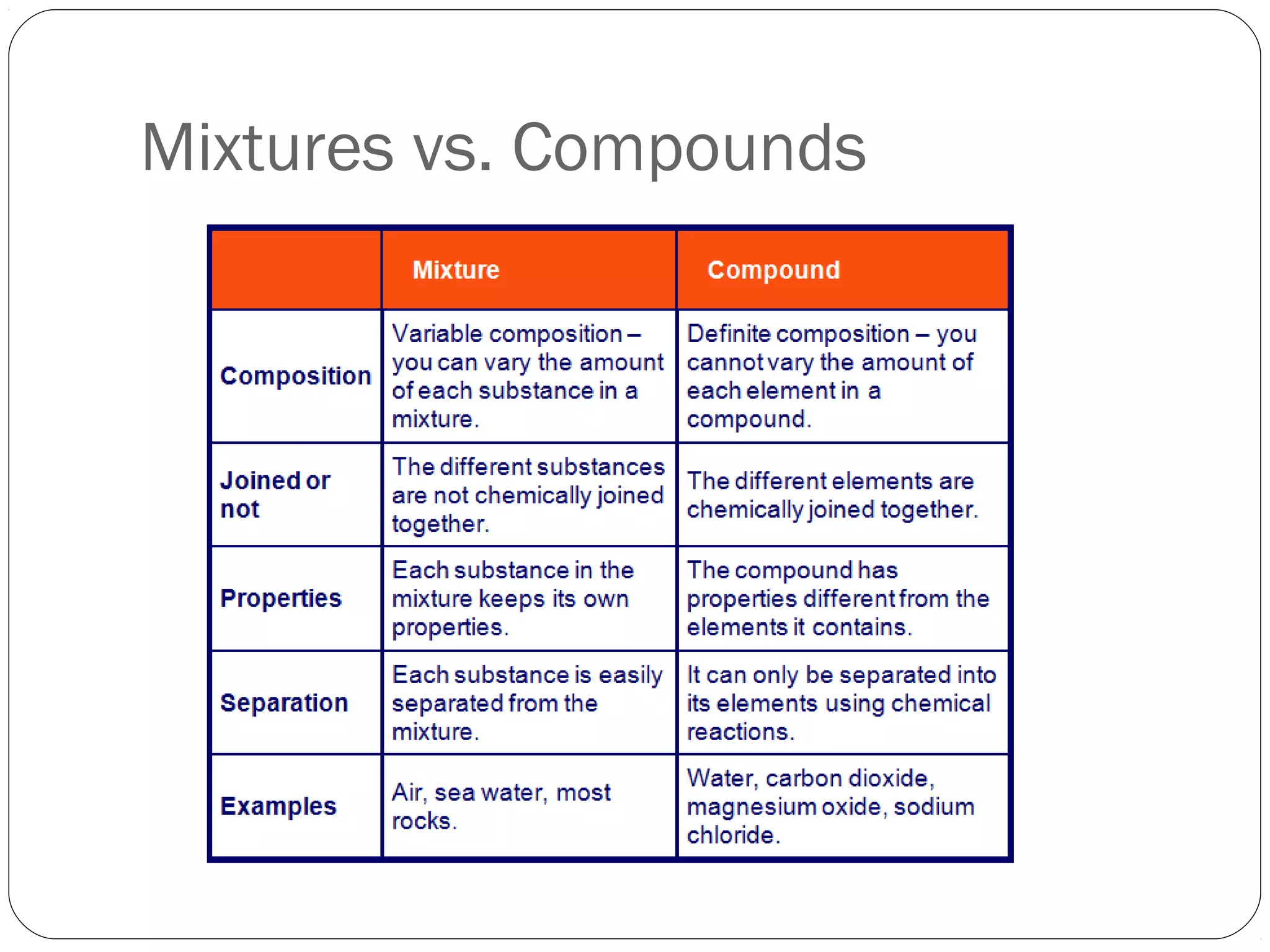
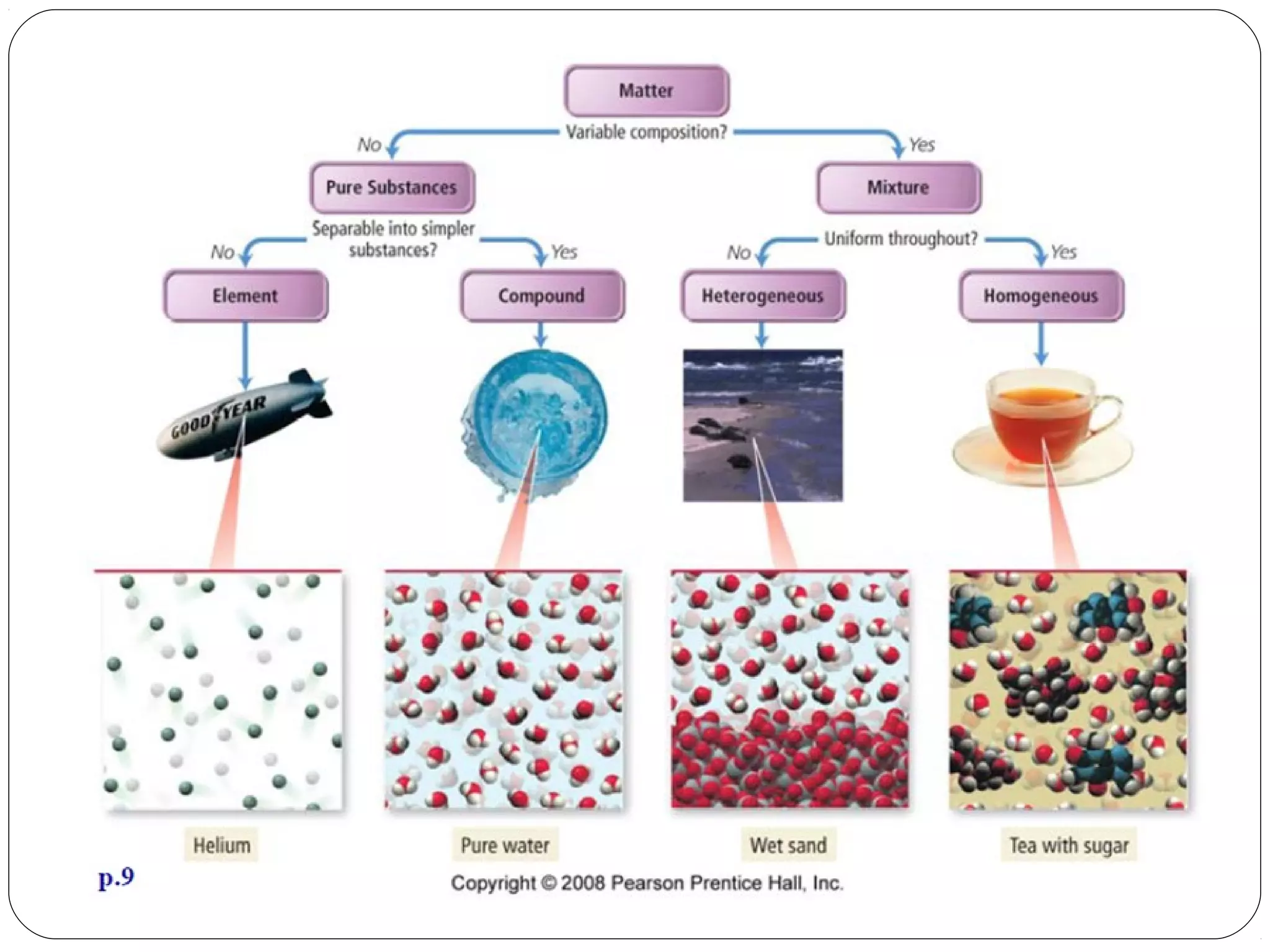
This document discusses the classification of pure substances and mixtures. It defines elements as pure substances that cannot be broken down further, compounds as pure substances made of two or more elements chemically bonded together, and mixtures as physical combinations of substances that are not chemically bonded and can be separated. Examples of each are given, such as copper being an element, salt being a compound of sodium and chlorine, and a salad being a mixture of various ingredients. Criteria for identifying elements, compounds and mixtures are outlined.