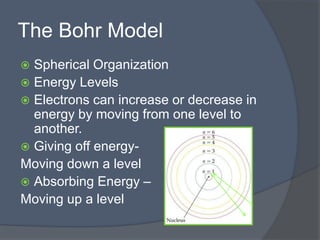
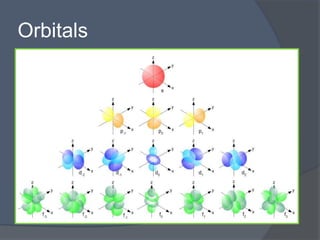
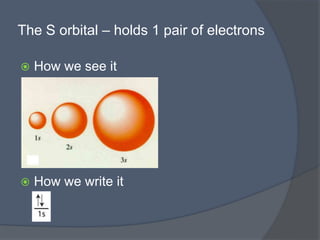
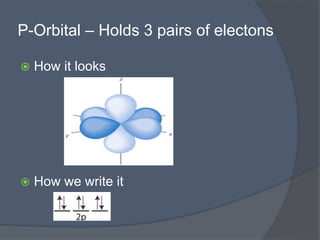
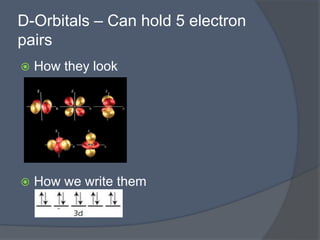
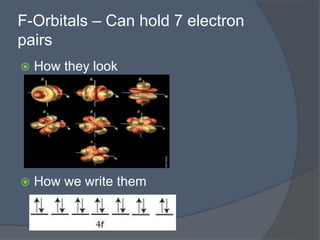
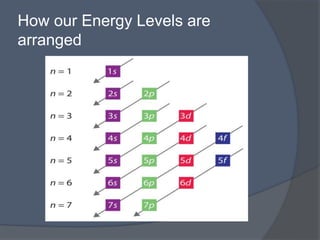
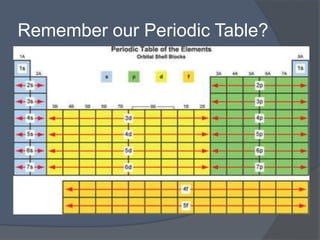
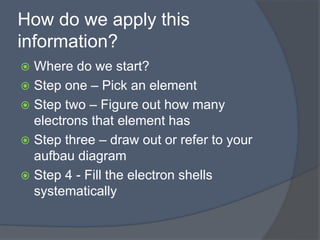
This document discusses electron configuration and how electrons are arranged in atoms. It introduces the Bohr model of electron shells and energy levels, then describes more accurate quantum mechanical models of electron orbitals. The document explains the Aufbau principle of filling orbitals from lowest to highest energy, the Pauli exclusion principle of maximum two electrons per orbital, and Hund's rule of filling orbitals within the same level. It outlines the shapes and notations of s, p, d, and f orbitals and how they relate to the periodic table and electron configuration notation.