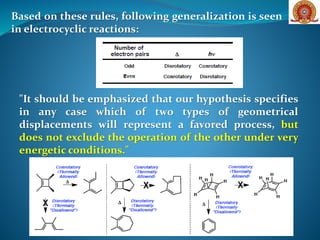
Electrocyclic reactions are pericyclic reactions where a conjugated polyene transforms into a cyclic compound, with mechanisms influenced by thermal or photochemical conditions. These reactions involve strict stereo-selectivity, governed by the number of π electrons and symmetry considerations in molecular orbitals, leading to allowed or forbidden processes depending on the charge of the reactants. Examples include various synthetic applications such as the thermal or photochemical cyclization of alkenes and the Nazarov cyclization, showcasing the reactions' utility in organic synthesis.














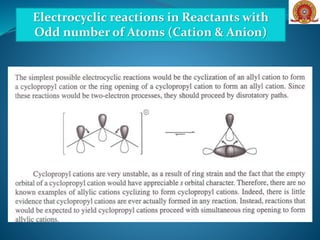




![Electrocyclic reactions in Reactants with
Odd number of Atoms (Cation & Anion)
[Formation and Cyclization of Dipolar Molecules]](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-21-320.jpg)
![Electrocyclic reactions in Reactants with
Odd number of Atoms (Cation & Anion)
[Formation and Cyclization of Dipolar Molecules]
The reaction
proceeds through
CONROTATORY
PROCESS](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-22-320.jpg)
![Electrocyclic reactions in Reactants with
Odd number of Atoms (Cation & Anion)
[Formation and Cyclization of Dipolar Molecules]](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-23-320.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-24-320.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-25-320.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]
STEREOSPECIFIC REACTIONS](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-26-320.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]
NON-STEREOSPECIFIC REACTIONS
1,3-Butadiene cyclize on photo-irradiation with UV light
with wavelengths above 220 nm to form the predicted
DISROTATORY ring closure products](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-27-320.jpg)
![Electrocyclic reactions
[Photochemical Cyclizations]
NON-STEREOSPECIFIC REACTIONS](https://image.slidesharecdn.com/electrocyclicreactions-200522152647/85/Electrocyclic-reactions-28-320.jpg)






