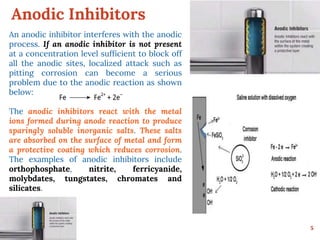
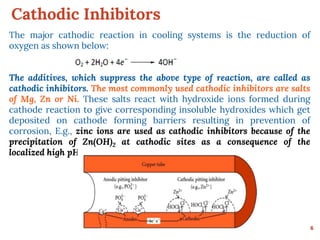
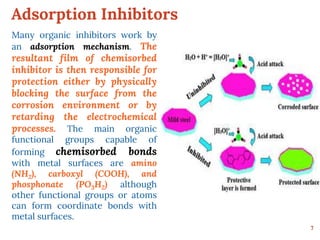
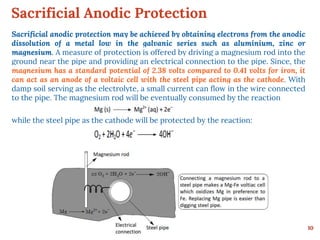
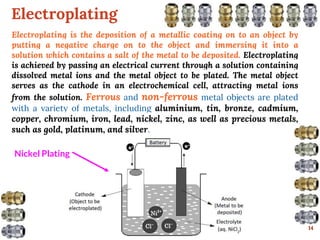
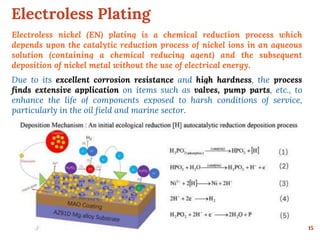
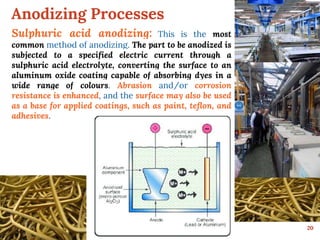
This document discusses various methods for preventing corrosion of metals. It begins by introducing the importance of preventing corrosion, which causes huge economic losses. The main methods discussed are modifying the material through coatings or alloys to increase corrosion resistance, using corrosion inhibitors, cathodic protection, and protective coatings. For coatings, it describes metallic coatings like electroplating, electroless plating and zinc coatings, as well as inorganic coatings like anodized aluminum coatings. It also discusses factors that affect the corrosion rate like the metal's purity, environment pH, and presence of impurities.














![23
References
The some contents are taken from:
Chemistry For Engineers
By
Harish Chopra
Anupama Parmar
[In addition, Internet sources have also been used]](https://image.slidesharecdn.com/corrosionprevention-200522152038/85/Corrosion-prevention-23-320.jpg)
