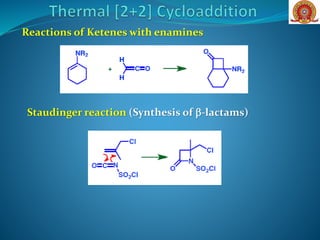
The document discusses cycloaddition reactions, which are pericyclic processes leading to the formation of rings from two conjugated systems. It covers various types of cycloaddition reactions, including [2+2], [4+2] (Diels-Alder), and [1,3]-dipolar cycloaddition, along with concepts like suprafaciality and antarafaciality that impact reaction feasibility. Additionally, it explores specific reactions involving enones and alkenes, with practical applications in synthesizing antifungal agents and other compounds.

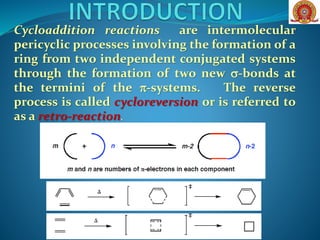
![Cycloaddition reactions can be discussed under
three important class of reactions
[2+2] Cycloaddition
[4+2] Cycloaddition [Diels-Alder reaction]
[1,3]-Dipolar cycloaddition.](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-3-320.jpg)
![Thermally, [2 + 2] cycloaddition is geometrically
forbidden, as the HOMO and LUMO of the
participating olefins would not be able to
achieve the orbital overlap required for σ-bond
formation.](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-4-320.jpg)
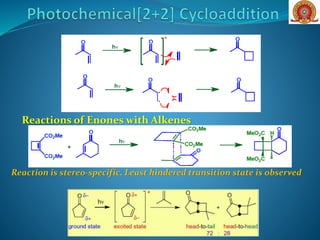
![On the other hand, the photochemical [2 + 2]
cycloaddition is allowed and leads to
stereospecific cyclobutane formation.](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-5-320.jpg)
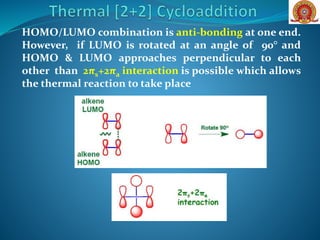
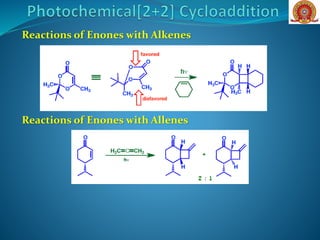
![If a closer look is taken about the thermal [2+2]
cycloaddition, as it is geometrically forbidden, but not
orbital symmetry forbidden.
It can be explained on the basis of two new concepts,
suprafaciality and antarafaciality, The consequence of
suprafaciality and antarafaciality is that many
processes that are Woodward-Hoffmann allowed can
be forbidden to occur because of geometrical
constraints on the system
Suprafaciality- when, in a
pericyclic reaction, the bond
forming interaction occurs on
the same face of a π-system,
Antarafaciality- when, in a
pericyclic reaction, the bond
forming interactions occur on
opposite faces of a π-system.](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-6-320.jpg)
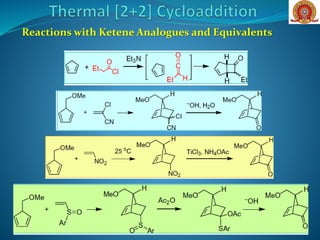
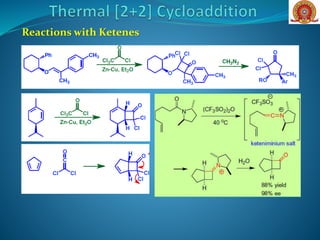
![Removal of steric bulk (H-
atoms) around the π-system
(as in the ketene) allows
antarafacial bond formation
that is geometrically
forbidden in the ethylene [2
+ 2].](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-7-320.jpg)

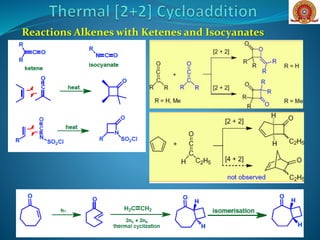
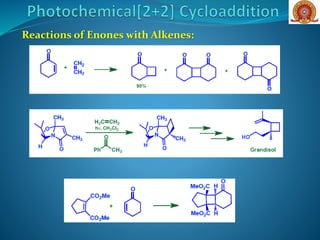
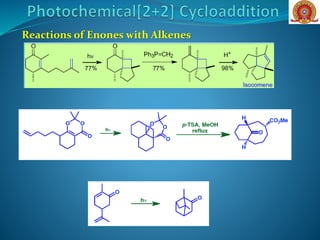
![Reactions of Enones with Alkenes: The first step in Corey’s
synthesis of caryophyllene involved addition of cyclohexenone
to isobutene to give predominantly the trans-cyclobutane (head-
to-tail) derivative
Major Product
O
H
H
Cycloaddition of an enone with a cyclic alkene (A) [cyclobut-1-
ene-1-carbonitrile], can occur with good stereoselectivity in
favour of the thermodynamically more stable exo
diastereoisomer O
NC
O
H
CNH
hv
DCM
+
(A)](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-10-320.jpg)
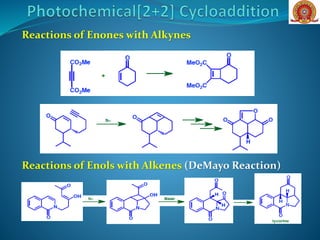
![Addition of C=C and C=O [Paterno-Buchi Reaction]
If an aldehyde or ketone -system replaces one of the alkene
units, then photochemical [2+2] cycloaddition is termed the
Paterno–Buchi reaction and an oxetane product is produced.
Typically a mixture of stereo- and regioisomers of the oxetane
is formed in intermolecular cycloadditions of this type,
although the use of small-ring alkenes favours the cis-fused
ring products.
Reactions are REGIOSELECTIVE](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-15-320.jpg)
![Addition of C=C and C=O [Paterno-Buchi Reaction]
Synthesis of the antifungal agent (+)-preussin, cycloaddition
of benzaldehyde with the dihydropyrrole led to the cis-fused
products [A] and [B]. Hydrogenolysis of the benzylic C–O
bond and reduction of the carbamate of the diastereomer [A]
gave the target compound preussin.
Preussin
[A] [B]](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-16-320.jpg)
![Addition of C=C and C=O [Paterno-Buchi Reaction]
Photocycloaddition of furan with nonanal gave the exo
product [C], which was converted to the antifungal
metabolite avenaciolide.
3,4-Dimethylfuran and (3-Benzyloxy)-propanal undergoes
Paterno-Buchi reaction to give oxetane derivative [D].
3,4-Dimethylfuran (3-Benzyloxy)-propanal [D]
Avenaciolide
[A]](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-17-320.jpg)
![Miscellaneous Reactions
bicyclo[2.2.1]hepta-2,5-diene](https://image.slidesharecdn.com/cycloadditionreactions22-200522152644/85/Cycloaddition-reactions-2-2-18-320.jpg)