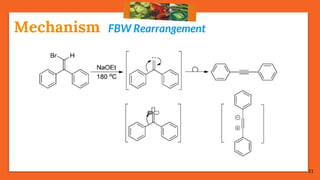
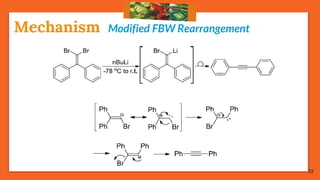
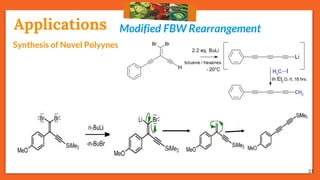
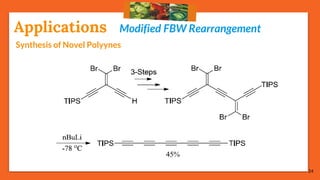
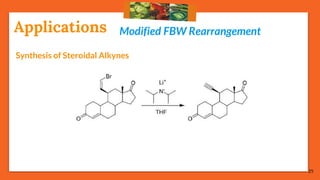
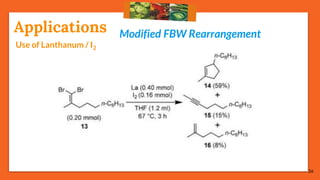
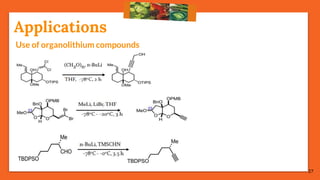
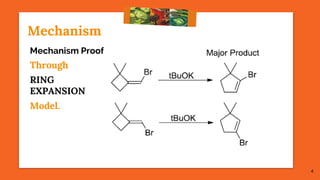
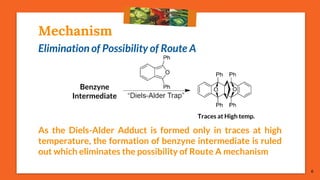
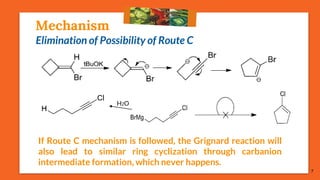
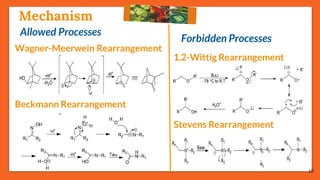
The Fritsch-Buttenberg-Wiechell rearrangement is a chemical reaction that transforms a 1,1-diaryl-2-bromo-alkene into a 1,2-diaryl-alkyne via a strong base, with detailed mechanisms discussed. Various pathways and their thermodynamic feasibility are analyzed, noting that the preferred reaction route is a stepwise trans migration process rather than concerted mechanisms. Applications of the modified rearrangement include the synthesis of novel polyynes and steroidal alkynes.

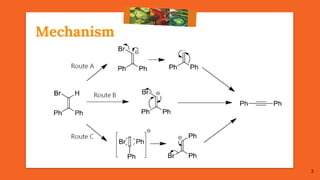
![Mechanism
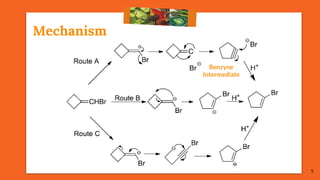
Only Possibility of Route B
[through Anionic 1,2-Sigmatropic Rearrangement]
But, as per Woodward-Hoffmann symmetry rule, the anionic 1,2-
Sigmatropic rearrangement is 4-electron process and is
FORBIDDEN.
8](https://image.slidesharecdn.com/fbwrearrangement-200522154352/85/Fbw-rearrangement-8-320.jpg)
![Mechanism
Orbital Symmetry conversion in a pericyclic reaction
[Woodward-Hoffmann Rule]
9
1,2-Sigmatropic
rearrangement may
be symmetrically
FORBIDDEN but
thermodynamically it
is a ALLOWED
process](https://image.slidesharecdn.com/fbwrearrangement-200522154352/85/Fbw-rearrangement-9-320.jpg)
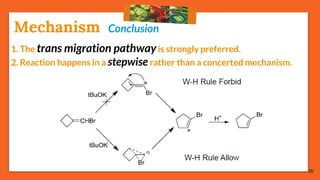
![14
If reaction follows
Route B,
it must have:
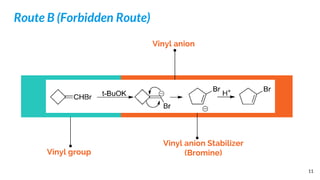
Strong Base
Vinyl Anion
Halogen
Non-Polar Solvents
[A] Bromine attach to terminal carbon
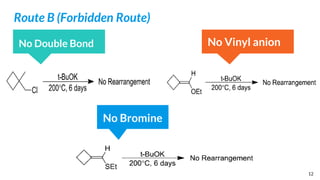
Route B (Forbidden Route):
[B] Bromine partially attach to vinyl group
Mechanism](https://image.slidesharecdn.com/fbwrearrangement-200522154352/85/Fbw-rearrangement-14-320.jpg)
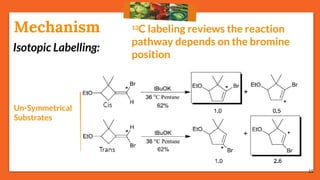
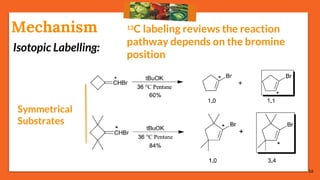
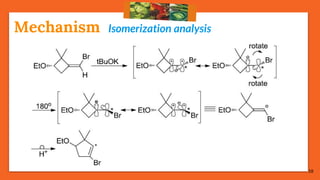
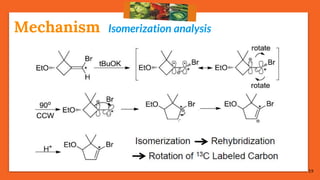
![17
Cis-Trans IsomerizationMechanism
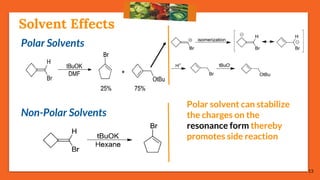
75% reactions prefer: Path [B], the migrating path
25% reactions prefer: Path [A], the re-hybridization path](https://image.slidesharecdn.com/fbwrearrangement-200522154352/85/Fbw-rearrangement-17-320.jpg)