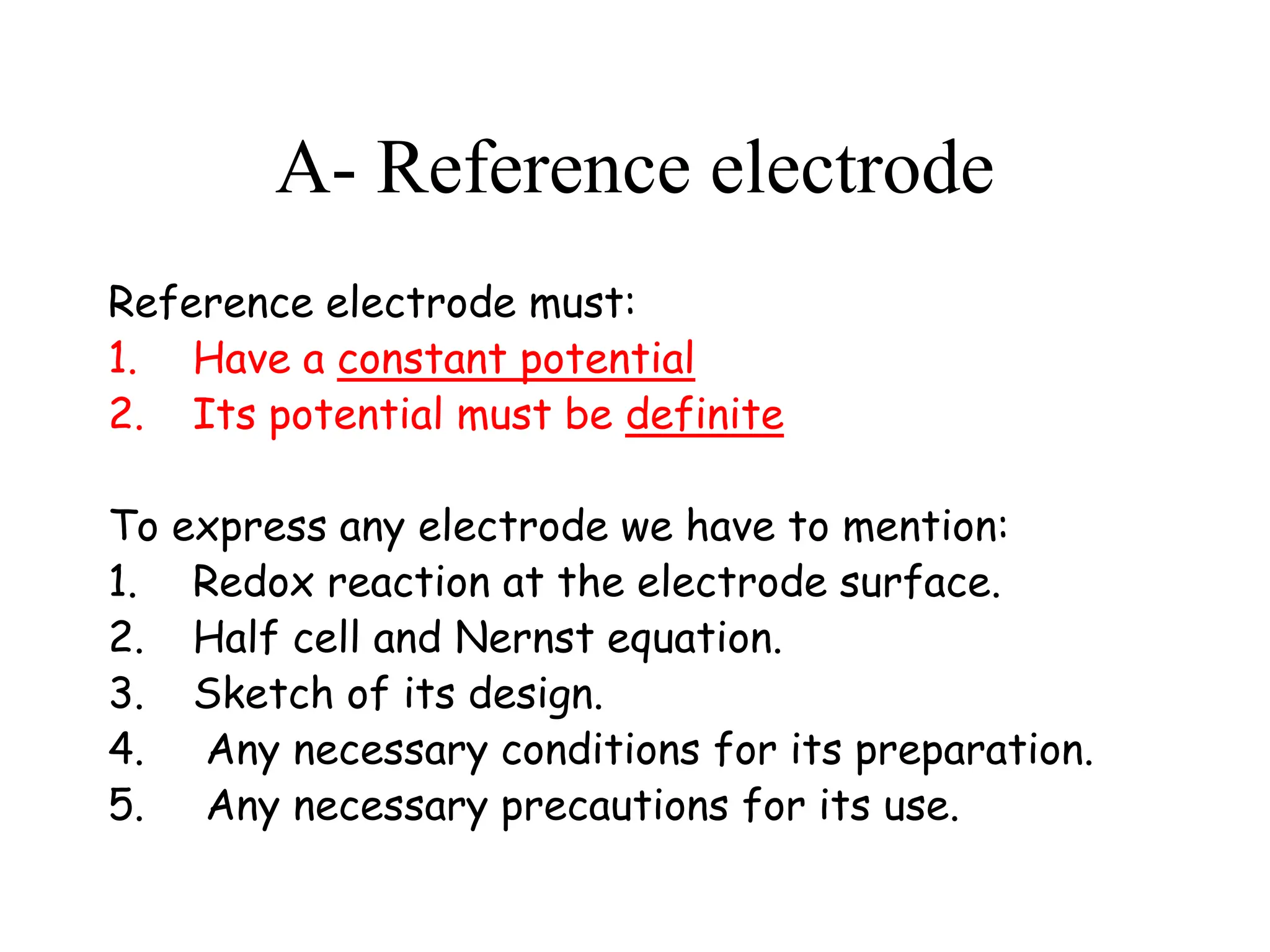
1. Electrochemistry involves studying chemical reactions that produce electricity or using electricity to cause non-spontaneous reactions.
2. Key concepts include galvanic cells which generate electricity from spontaneous redox reactions, electrolytic cells which use electricity to drive non-spontaneous reactions, and standard reduction potentials which quantify reaction tendencies.
3. Standard cell potentials allow calculation of cell voltage from half-reaction potentials under standard conditions.















![Galvanic Cells
The difference in electrical potential
between the anode and cathode is
called:
• Electromotive force emf
• Cell Voltage
• Cell potential
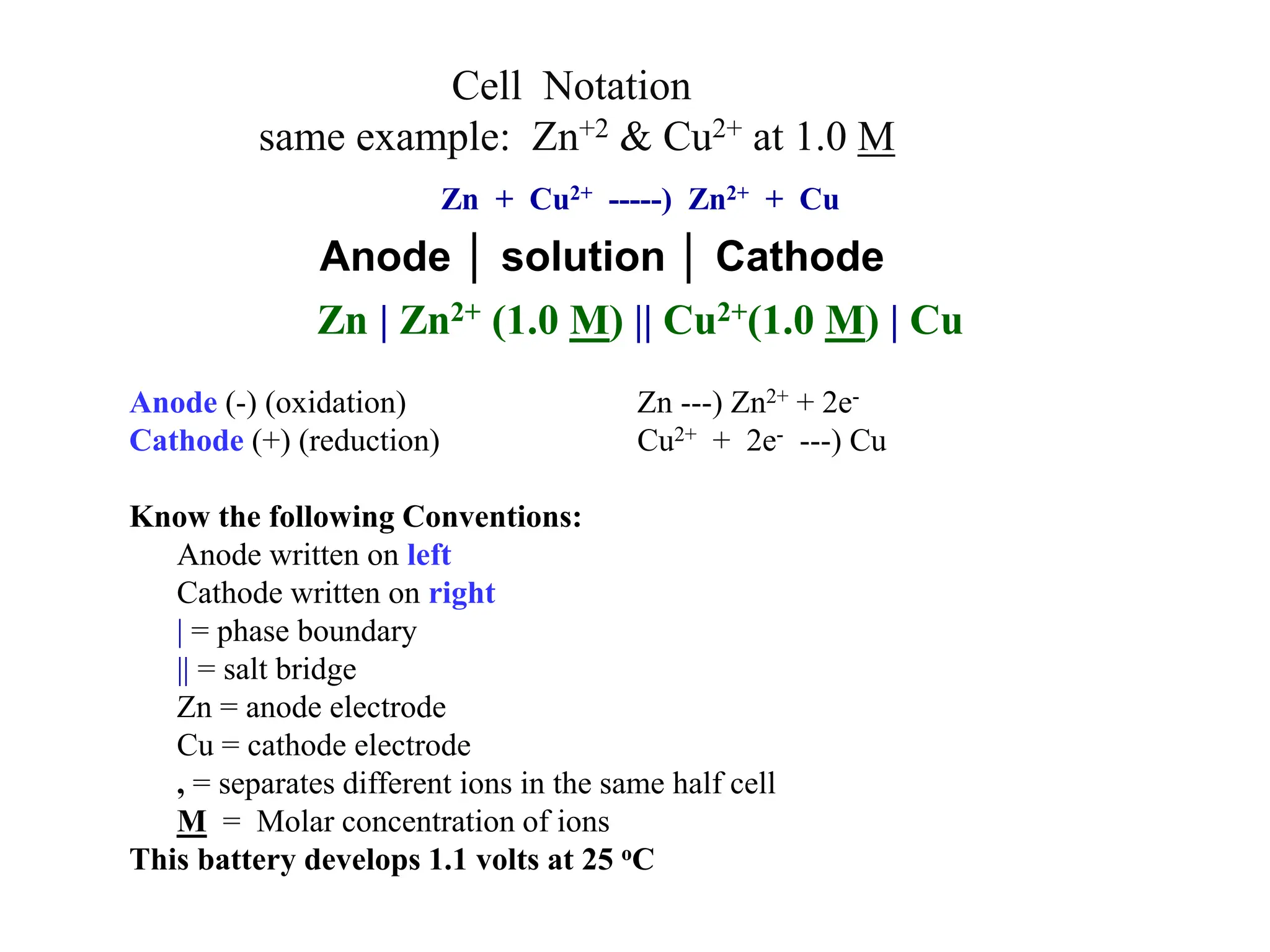
Cell Notation
Zn (s) + Cu2+ (aq) Cu (s) + Zn2+ (aq)
[Cu2+] = 1 M & [Zn2+] = 1 M
Zn (s) | Zn2+ (1 M) || Cu2+ (1 M) | Cu (s)
Anode │ solution │ Cathode](https://image.slidesharecdn.com/electrochemistryall-240203080728-890a1524/75/Electrochemistry-All-ppt-20-2048.jpg)









![Will the following reaction occur spontaneously at 250C if
[Fe2+] = 0.60 M and [Cd2+] = 0.010 M?
Fe2+ (aq) + Cd (s) Fe (s) + Cd2+ (aq)
2e- + Fe2+ Fe
Cd Cd2+ + 2e-
Oxidation:
Reduction:
n = 2
E0 = -0.44 – (-0.40)
E0 = __-0.04__ V
E0 = EFe /Fe – ECd /Cd
0 0
2+ 2+
-
0.0257
n
ln Q
E0
E =
-
0.0257
2
ln
-0.04 V
E =
0.010
0.60
E = __0.0126__ V
E > 0 _________________](https://image.slidesharecdn.com/electrochemistryall-240203080728-890a1524/75/Electrochemistry-All-ppt-36-2048.jpg)







![Calomel is the common name for the
compound Hg2Cl2.
electrode reaction in calomel half-cell
Eo = + 0.268V
E = Eo – (0.05916/2) log[Cl–]2
Temperature dependent
At 25 C , E = 0.244 V
Saturated calomel electrode (S.C.E.)](https://image.slidesharecdn.com/electrochemistryall-240203080728-890a1524/75/Electrochemistry-All-ppt-45-2048.jpg)
![Ag(s) | AgCl (sat’d), KCl (xM) | |
AgCl(s) + e = Ag(s) + Cl–
Eo = +0.244V
E = Eo – (0.05916/1) log [Cl–]
E (saturated KCl) = + 0.197 V (25oC)
Silver-silver chloride electrode](https://image.slidesharecdn.com/electrochemistryall-240203080728-890a1524/75/Electrochemistry-All-ppt-46-2048.jpg)





![a- Electrodes of the First Kind:
• The electrode system can be represented by M/Mn+, in which
the line represents an electrode – solution interface . For silver
electrode , we have
• Ag/Ag+
• the half – reaction is
• Ag+ + e = Ag(s) Eo = + 0.800V
E = 0.800 – (0.05916/1) log {1/[Ag+]}
Metallic electrodes](https://image.slidesharecdn.com/electrochemistryall-240203080728-890a1524/75/Electrochemistry-All-ppt-53-2048.jpg)



![Example:
Ag(s) | AgCl[sat’d], KCl[xM] | | Fe2+,Fe3+) | Pt
Fe3++e = Fe2+ Eo = +0.770V
Ecell = Eindicator – Ereference
= {0.770 – (0.05916/1) log [Fe2+]/[Fe3+]}
– {0.222 – (0.05916/1) log [Cl–]}
C. Electrodes of the third Kind Inert electrodes (Indicators electrodes for
redox reaction)
The inert metal used is usually platinum
The inert metal is contact with a solution containing the soluble
oxidized and reduced form of the redox half – reaction. “(Fe2+,Fe3+) “
And Ag/AgCl is used as ref. electrode:](https://image.slidesharecdn.com/electrochemistryall-240203080728-890a1524/75/Electrochemistry-All-ppt-57-2048.jpg)