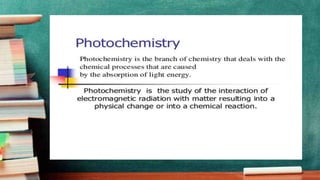
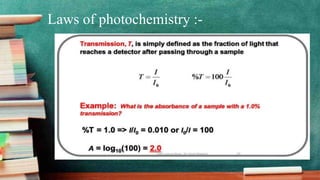
Photochemistry CECH-509 discusses photochemical reactions and the laws that govern them. Photochemical reactions are chemical reactions initiated by the absorption of light. Key points:
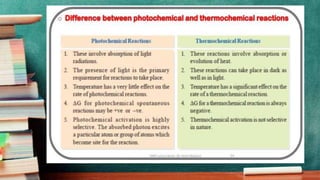
- Photochemical reactions increase free energy while thermal reactions decrease it. The rate of a photochemical reaction depends on the intensity of absorbed light.
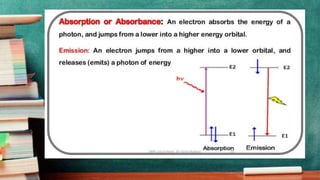
- Stark-Einstein law states that each reacting molecule absorbs a single photon, gaining energy to activate and enter the reaction.
- Quantum yield refers to the number of molecules reacted or formed per absorbed photon, indicating the reaction's efficiency. Values below 1 indicate a low yield while above 1 is high. Secondary reactions can result in yields above 1.