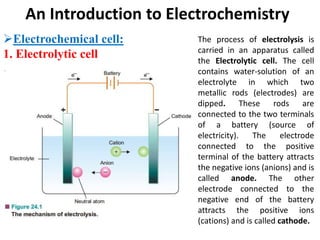
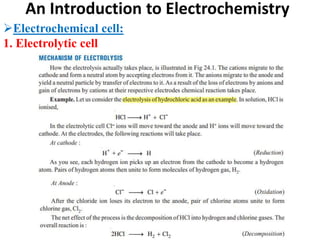
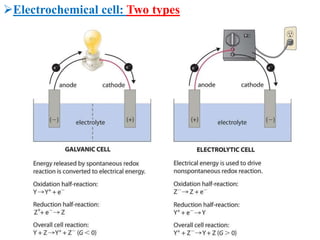
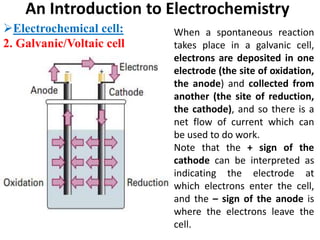
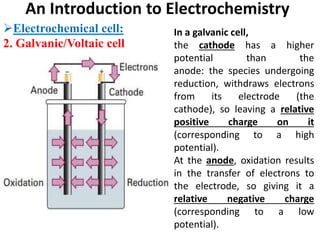
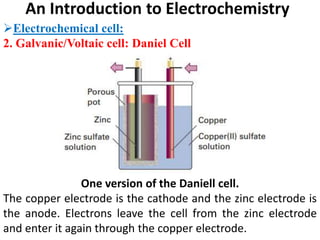
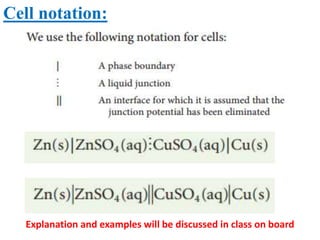
This document provides an introduction to electrochemistry and discusses electrochemical cells. It defines electrochemistry as the study of physical and chemical processes involving electrical energy. An electrochemical cell is a device that produces electrical work through a chemical reaction. There are two types of electrochemical cells: electrolytic cells and galvanic/voltaic cells. In an electrolytic cell, electricity is passed through an electrolyte to drive a non-spontaneous reaction. In a galvanic cell, a spontaneous reaction occurs producing electricity. The document discusses the components and examples of each type of cell. It also compares electrolytic and galvanic cells in terms of their similarities and differences.