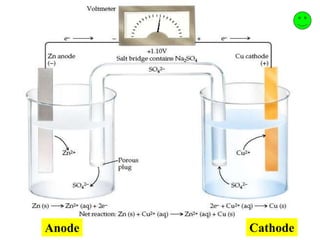
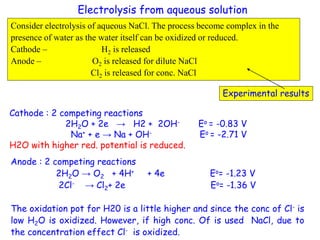
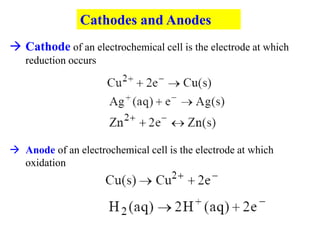
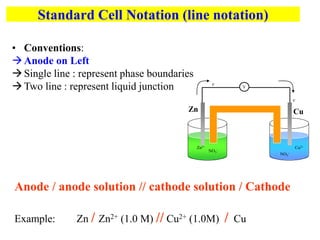
This document provides an introduction to electroanalytical chemistry. It discusses key concepts such as oxidation, reduction, and different types of electrochemical cells including galvanic and electrolytic cells. Examples of half-reactions and standard reduction potentials are given. The document also describes components of electrochemical cells such as electrodes, salt bridges, and electron flow. Diagrams illustrate electrolysis processes including the electrolysis of molten sodium chloride and aqueous sodium chloride. Standard cell notation for writing electrode reactions is also defined.