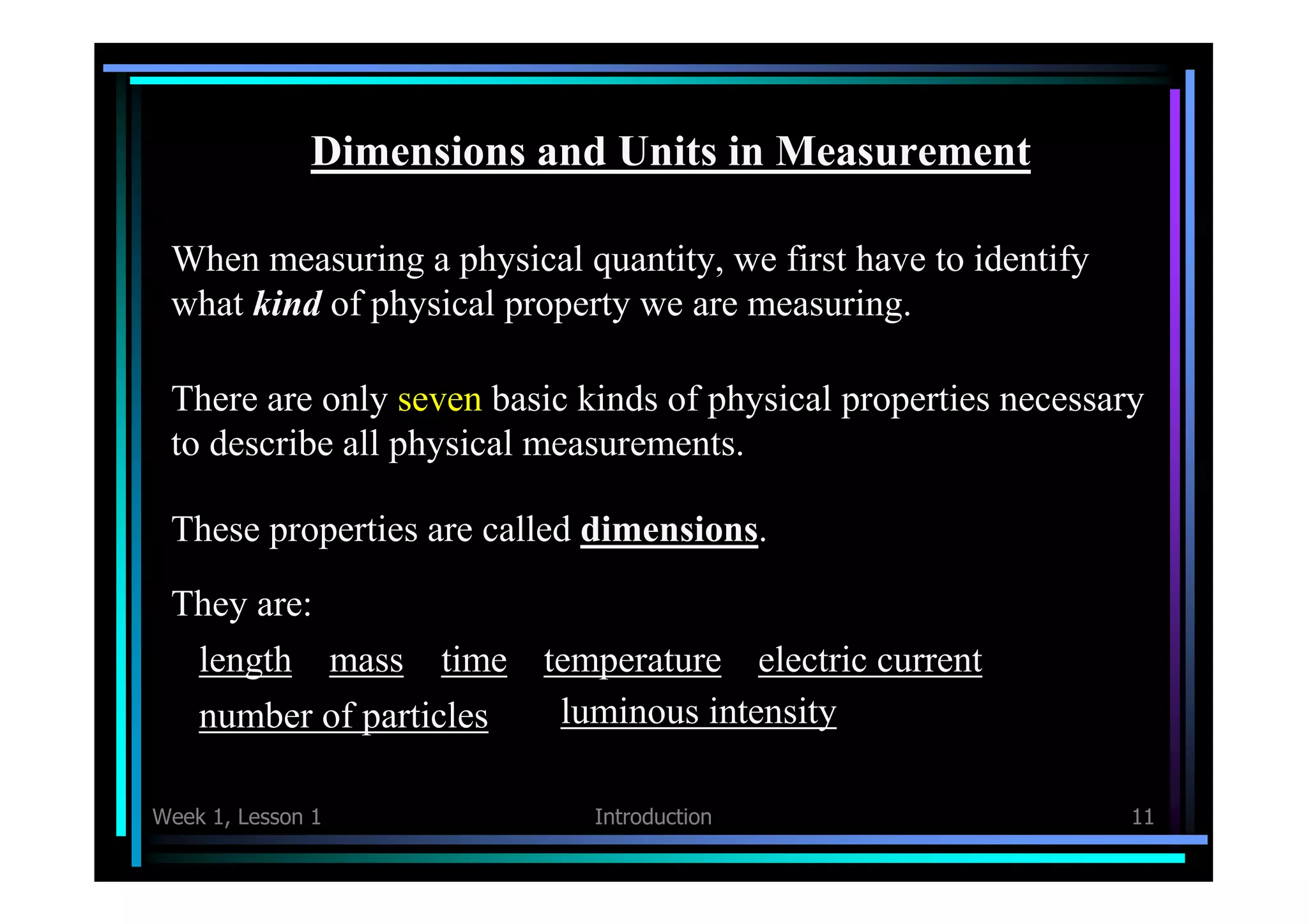
This document provides an introduction to the topics that will be covered in Week 1, Lesson 1 of a physics course. It includes brief descriptions of what physics is, how measurements are made and quantified in terms of accuracy and precision, the fundamental dimensions and units used in physics, how to perform calculations and conversions involving units, and how to determine the appropriate number of significant figures in measurements and calculations. The key topics covered are counting, measuring, accuracy vs precision, dimensional analysis, unit conversions, and significant figures. Students are assigned to read sections 1.1 to 1.6 in their textbook to prepare for the lesson.