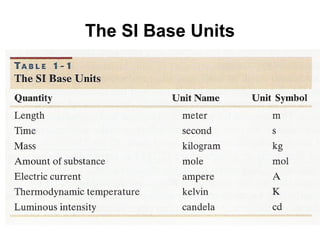
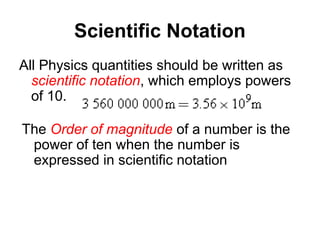
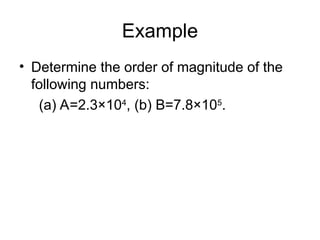
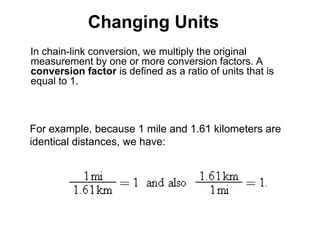
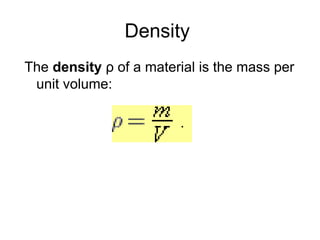
This document discusses the fundamental concepts of physics related to measurement, including the definition of physical quantities, units, and the International System of Units (SI). It covers basic measurements such as length, time, and mass, and explains important principles like significant figures, conversions, and the precision of measurements. The document includes exercises for practical application of these concepts to enhance understanding.