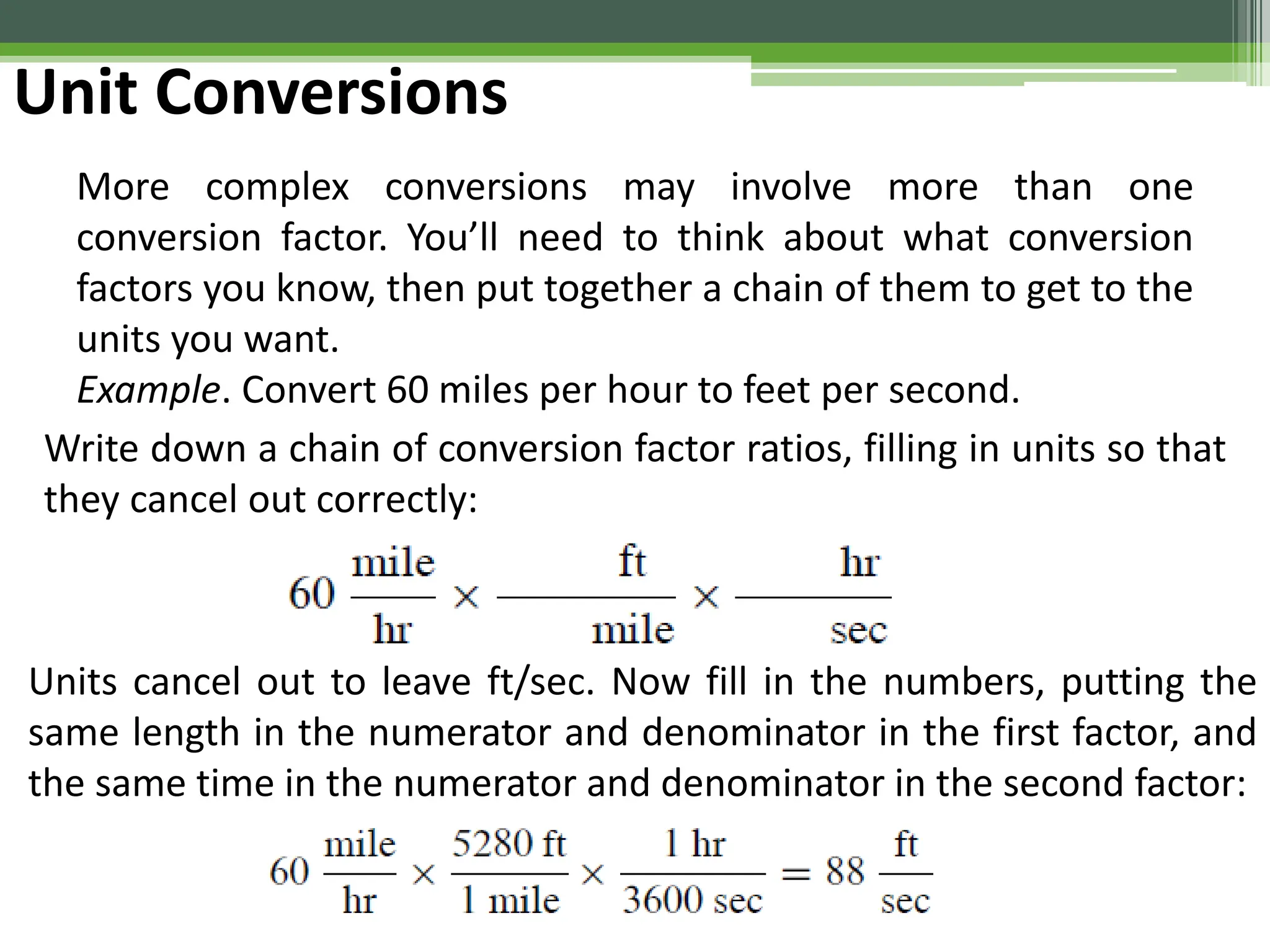
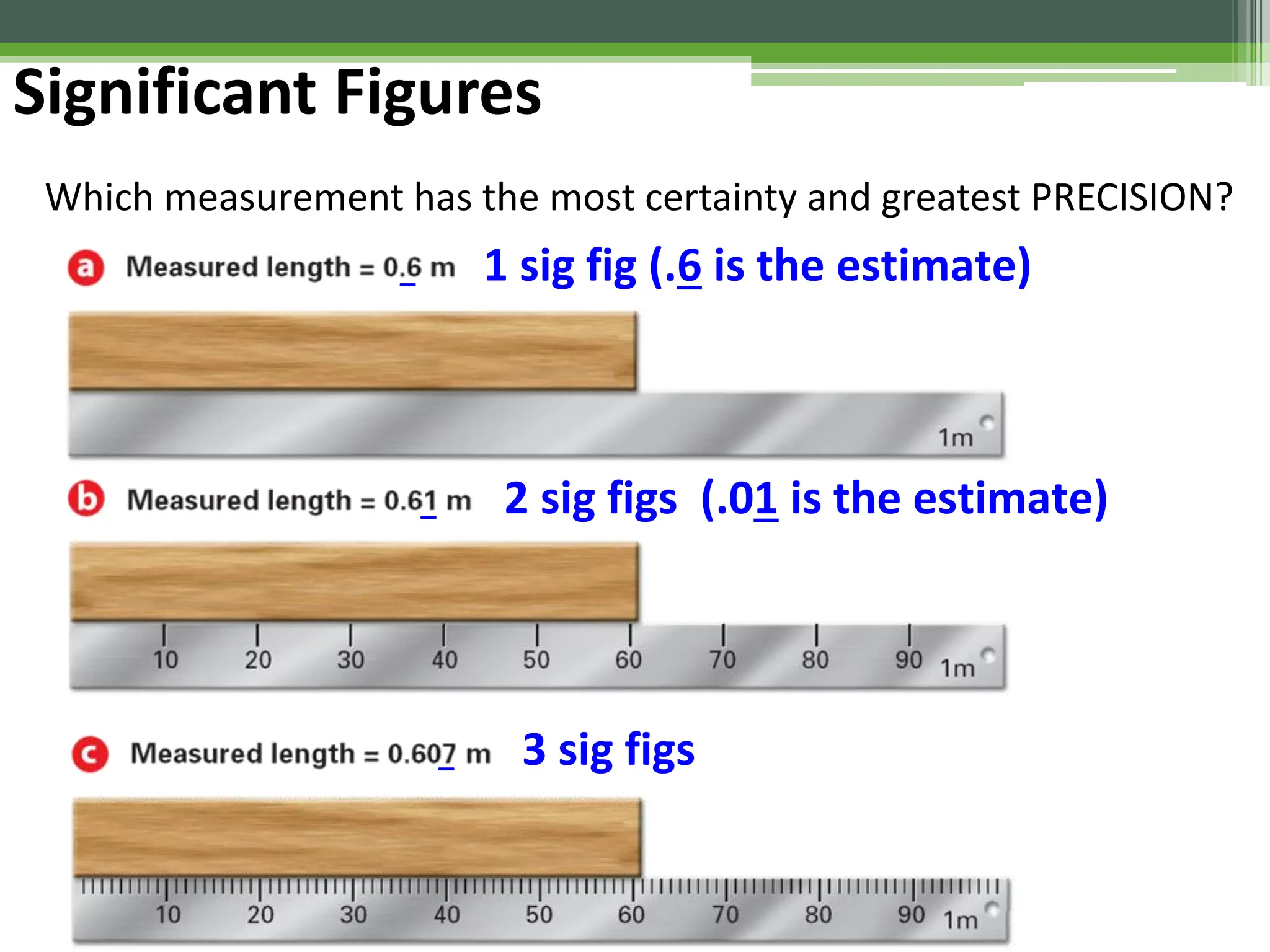
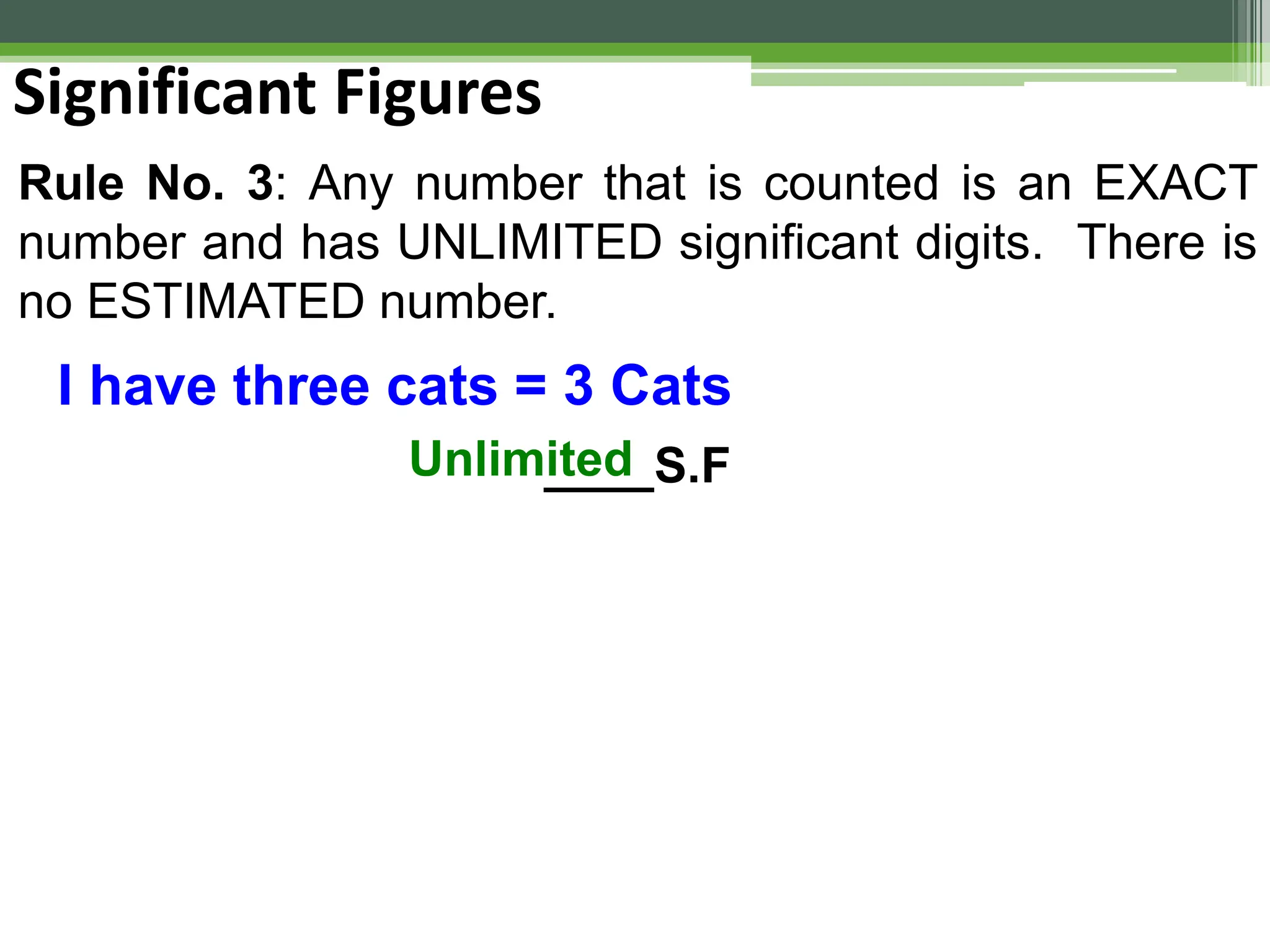
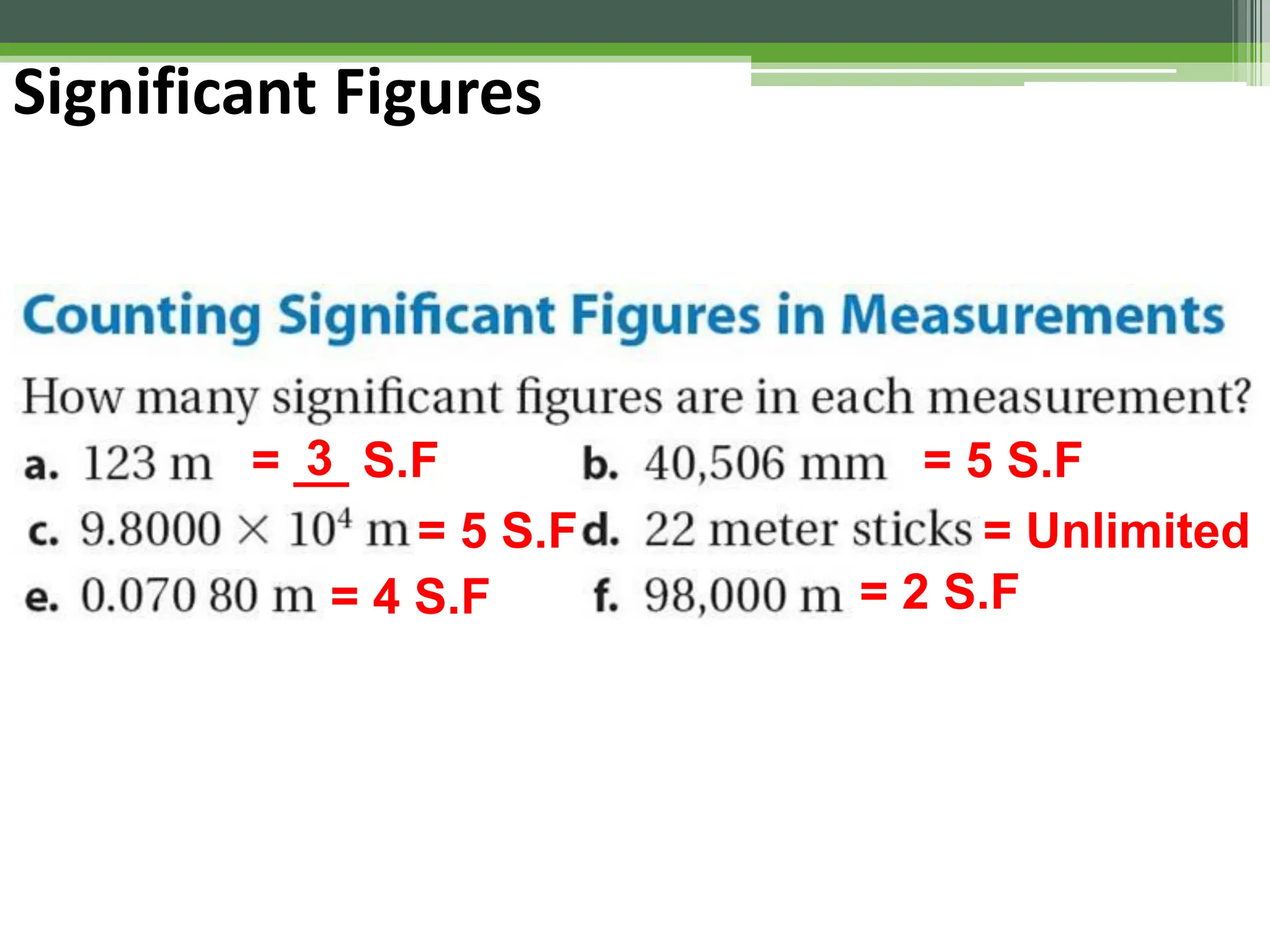
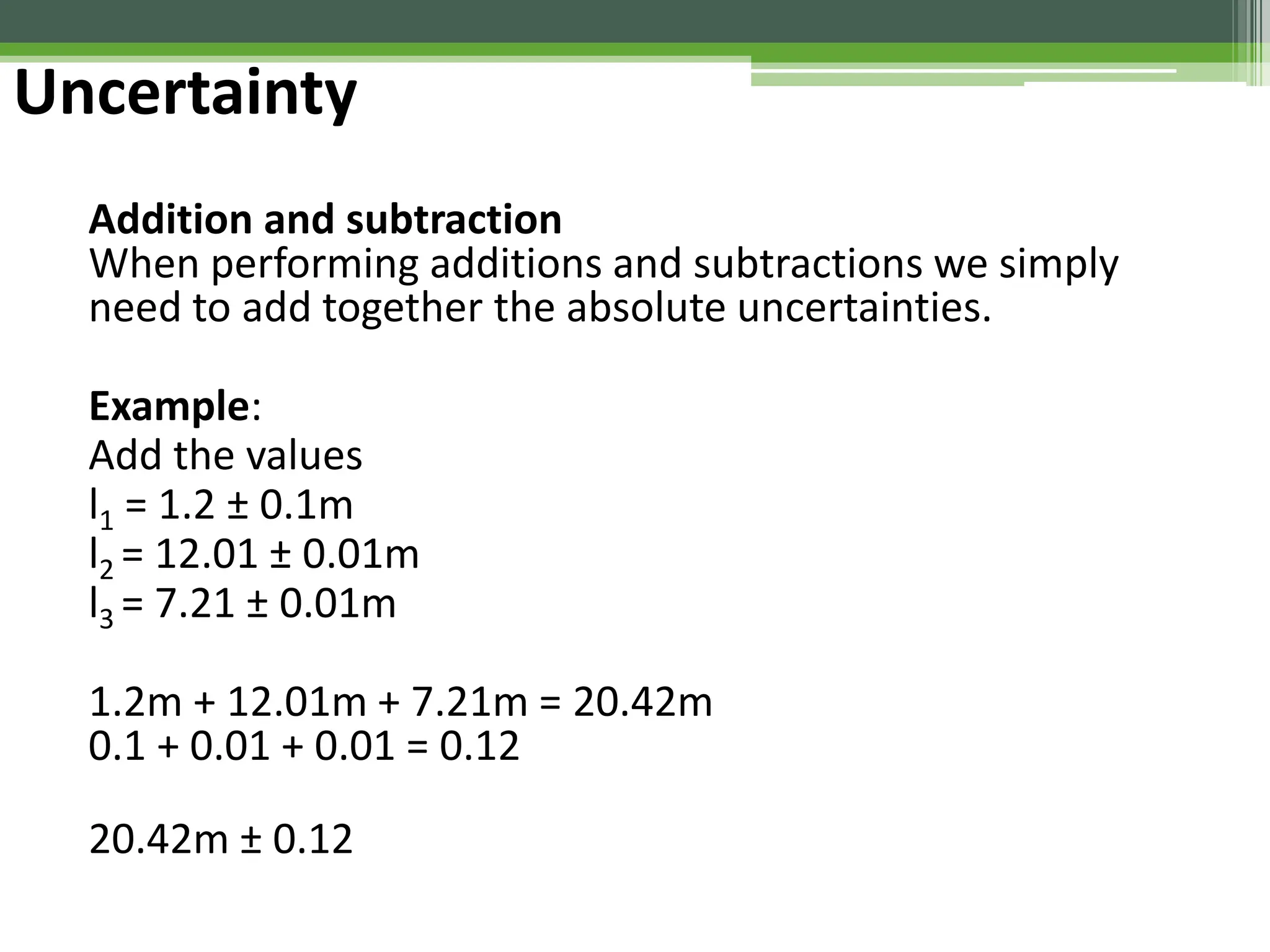
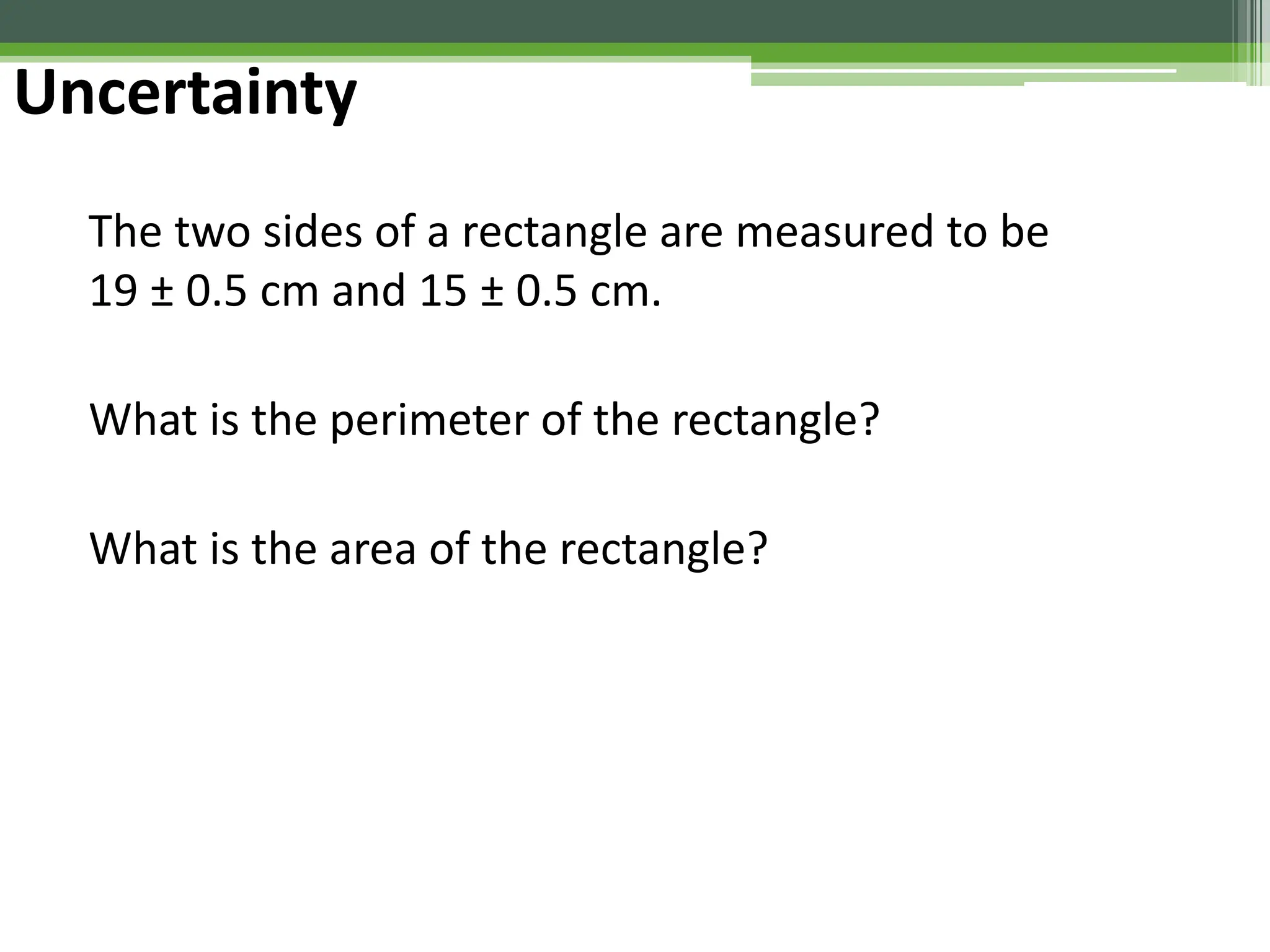
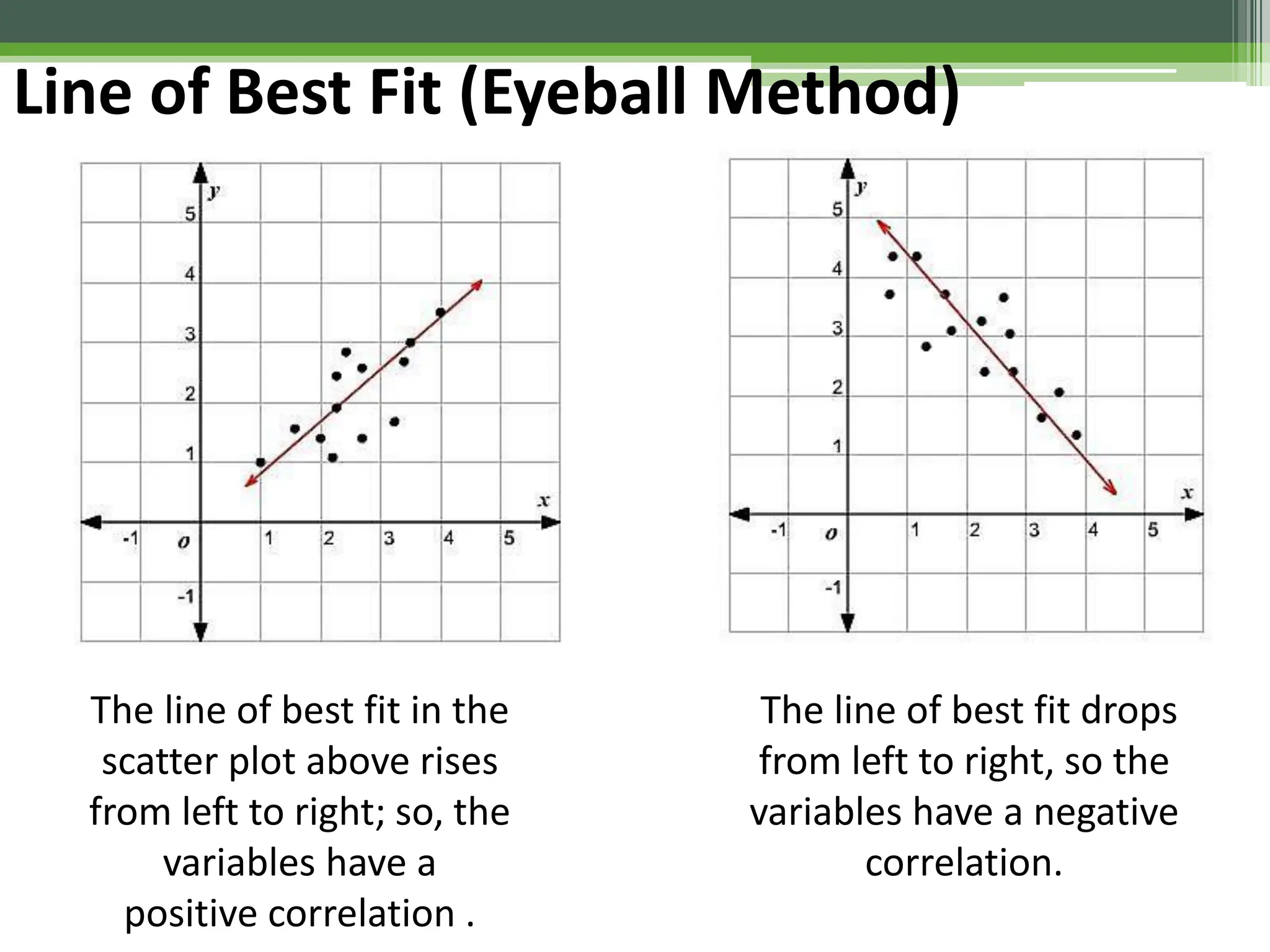
The document outlines foundational topics in general physics including the definitions of physical units, systems of measurement, and unit conversions. It emphasizes the importance of accuracy and precision in measurements, detailing the concepts of random and systematic errors, least count, significant figures, and uncertainty. Additionally, it introduces statistical methods for analyzing data, such as the line of best fit in graphical presentations.