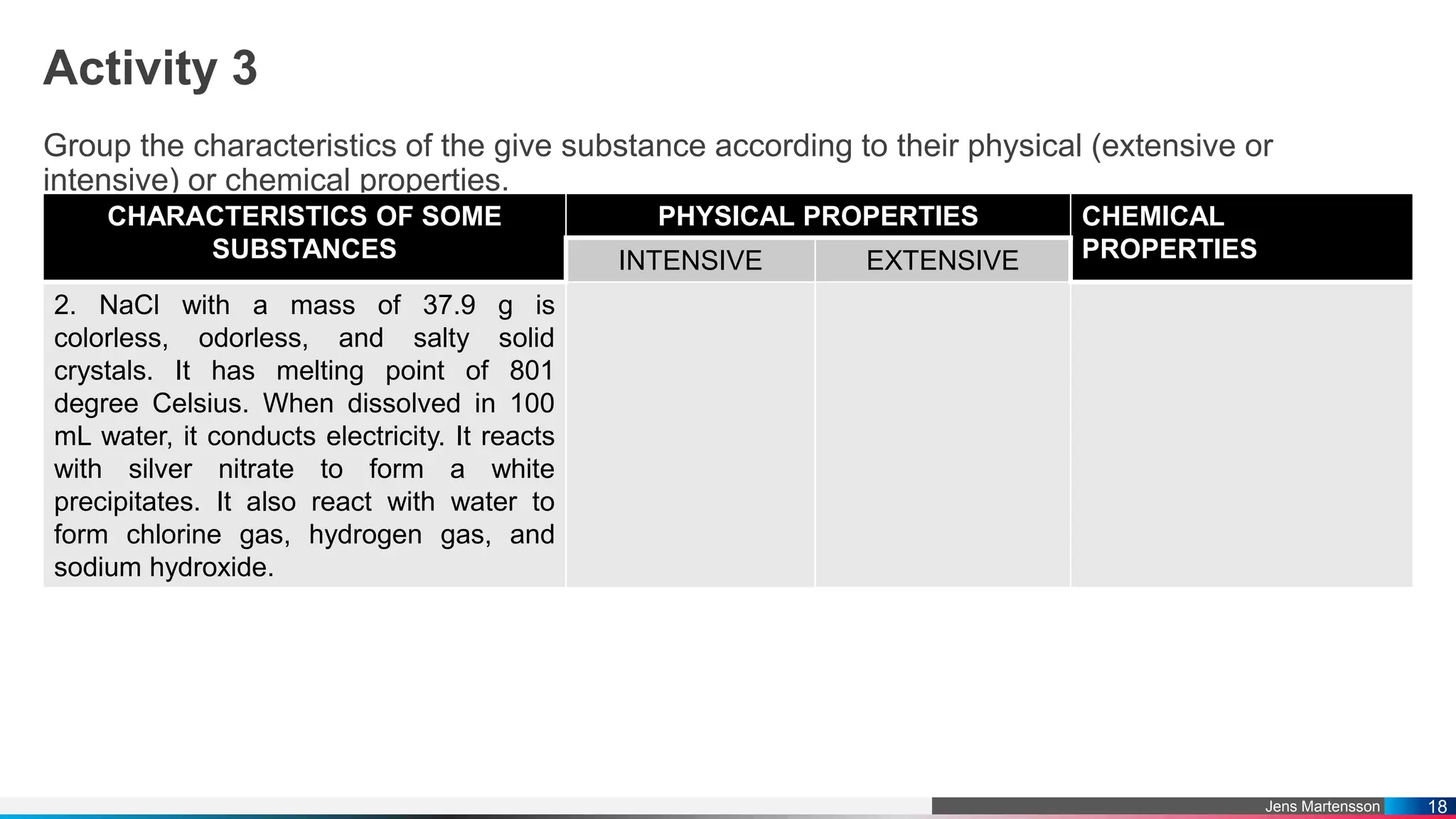
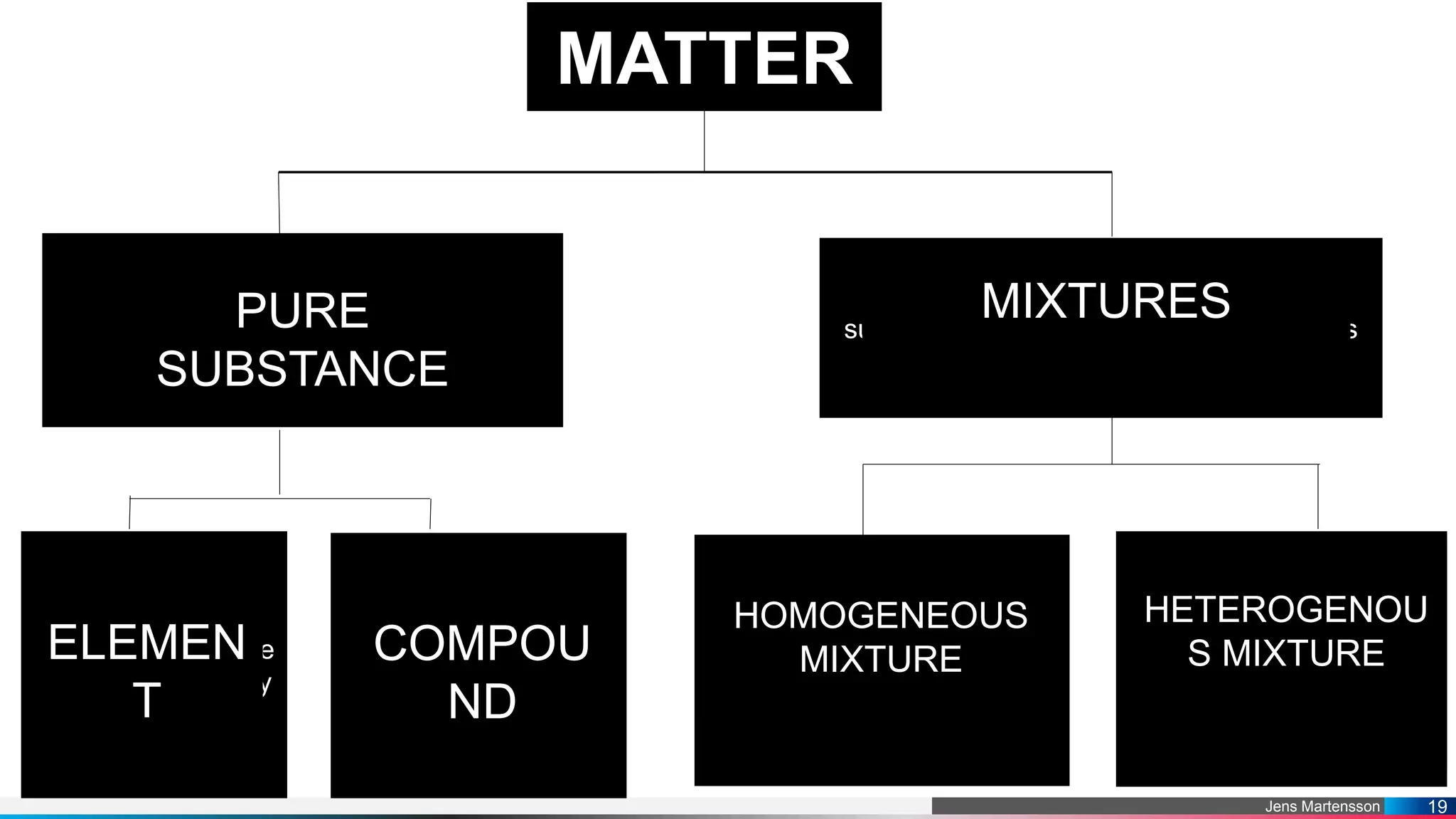
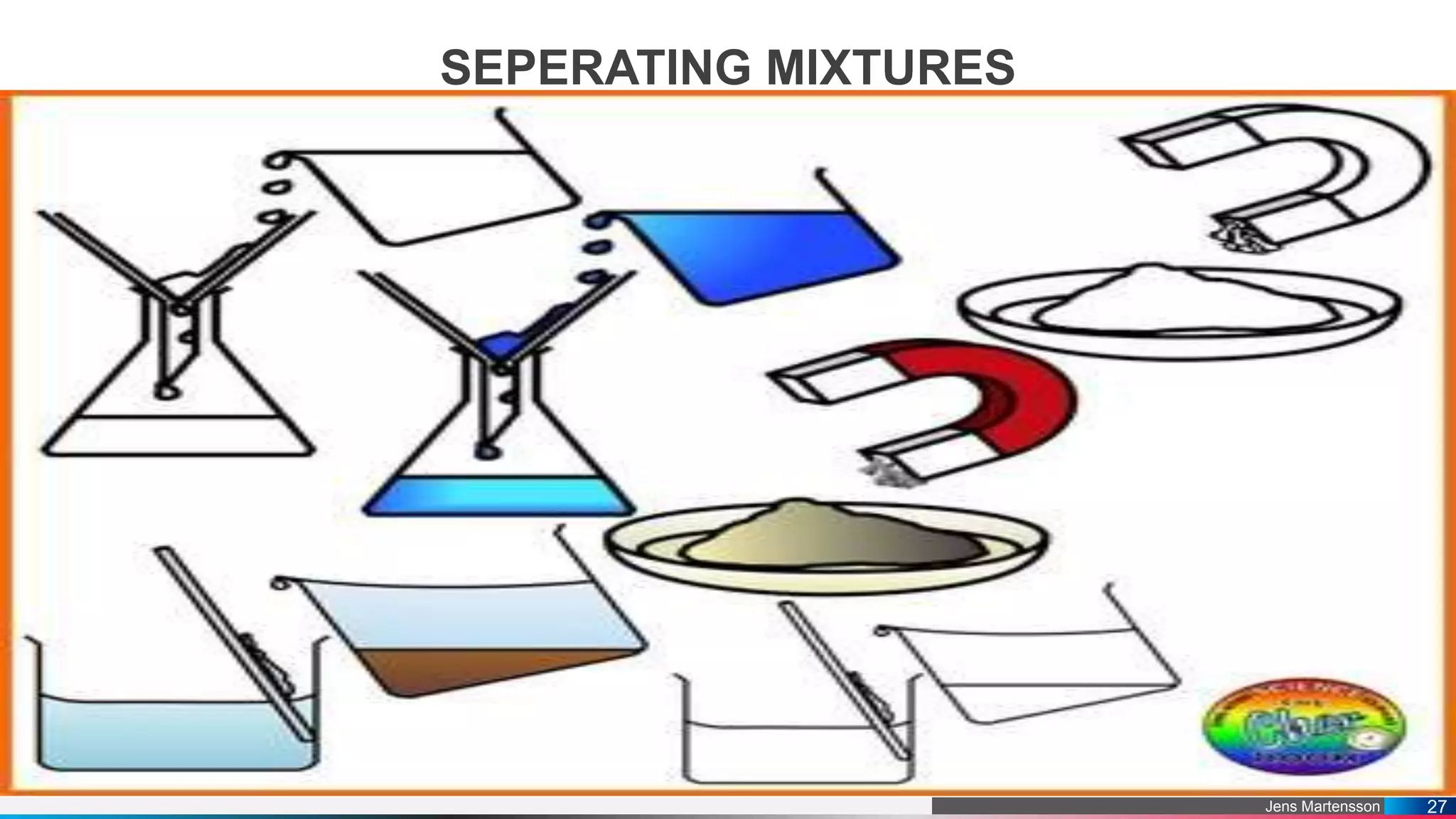
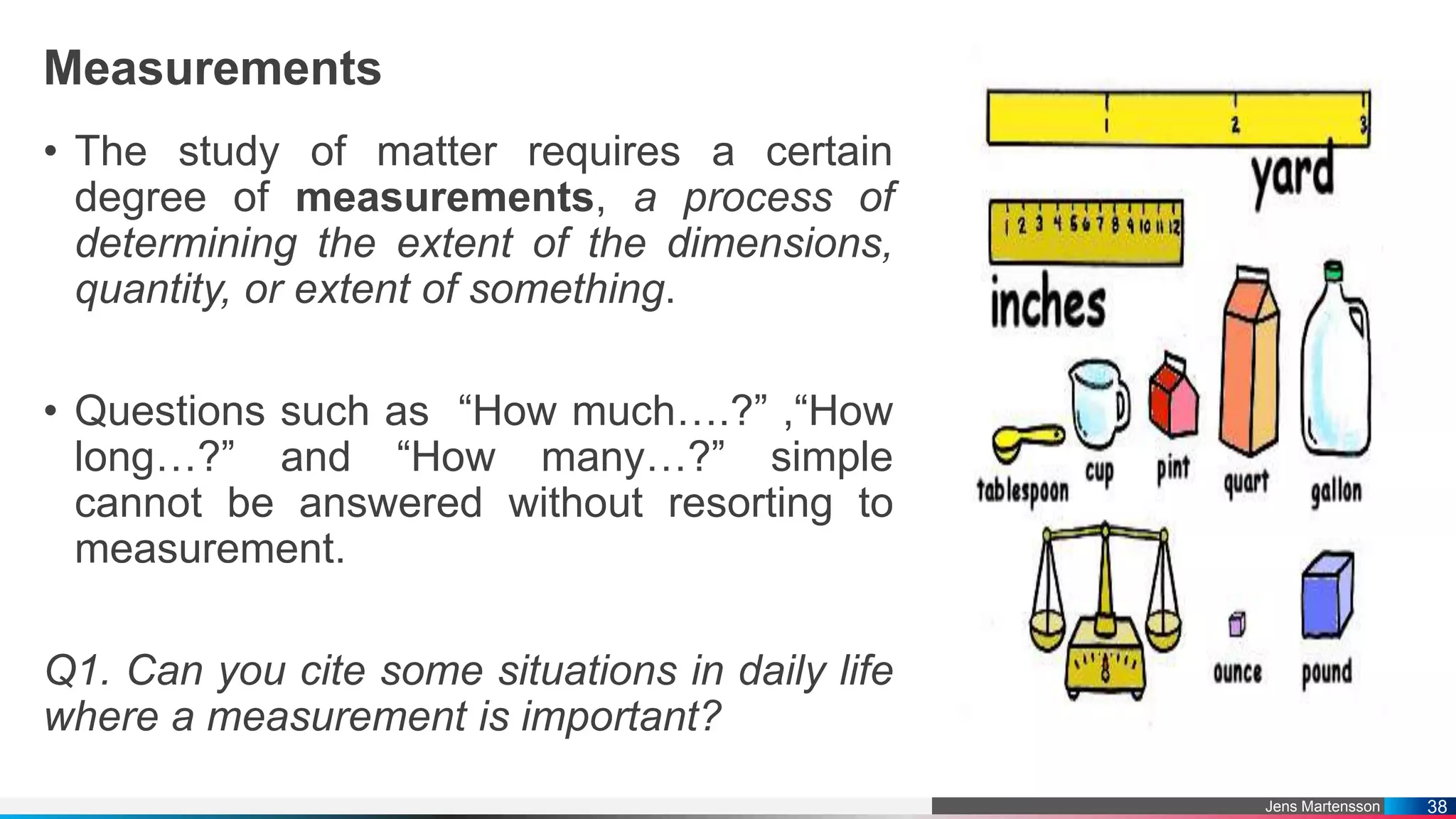
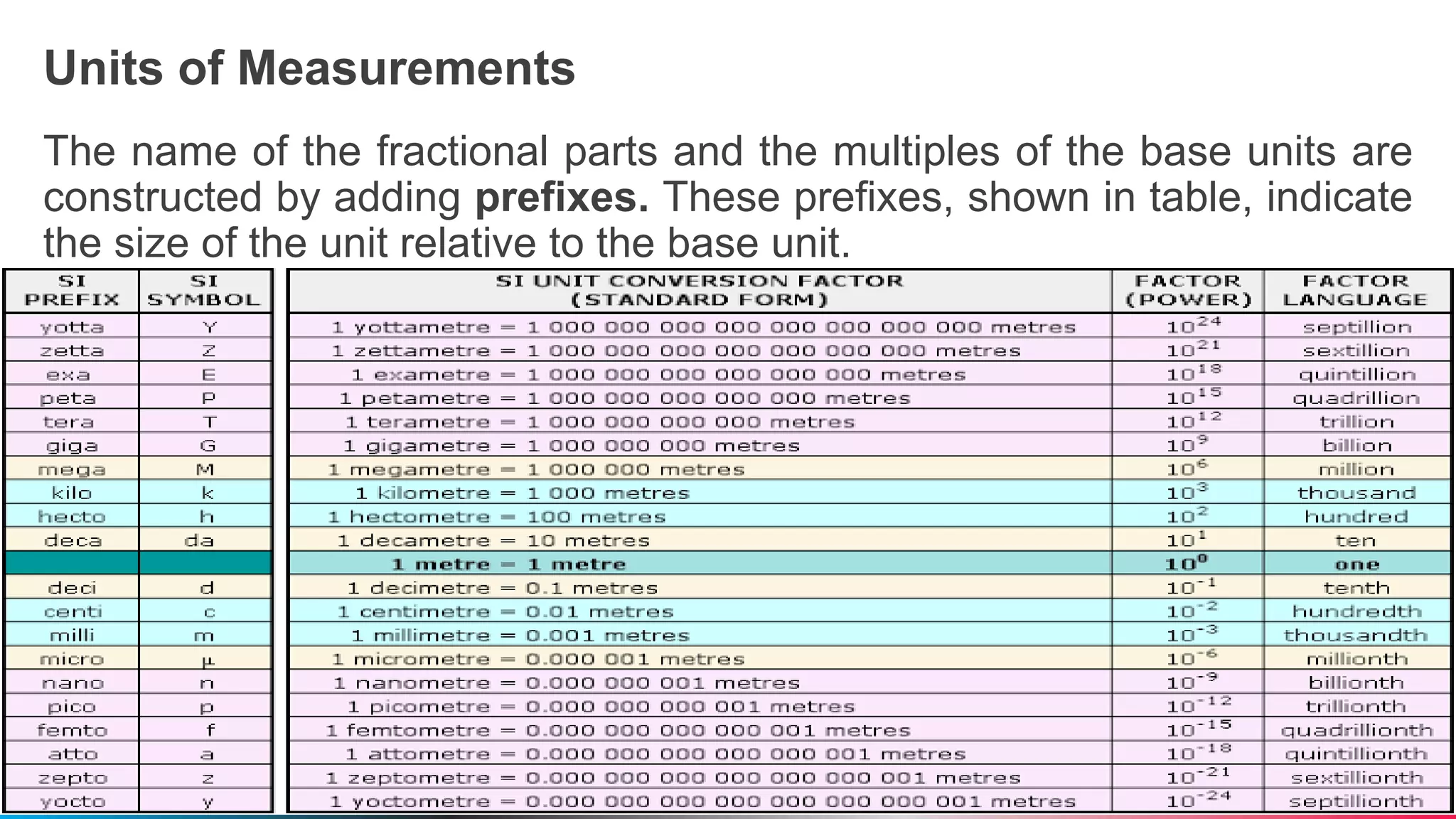
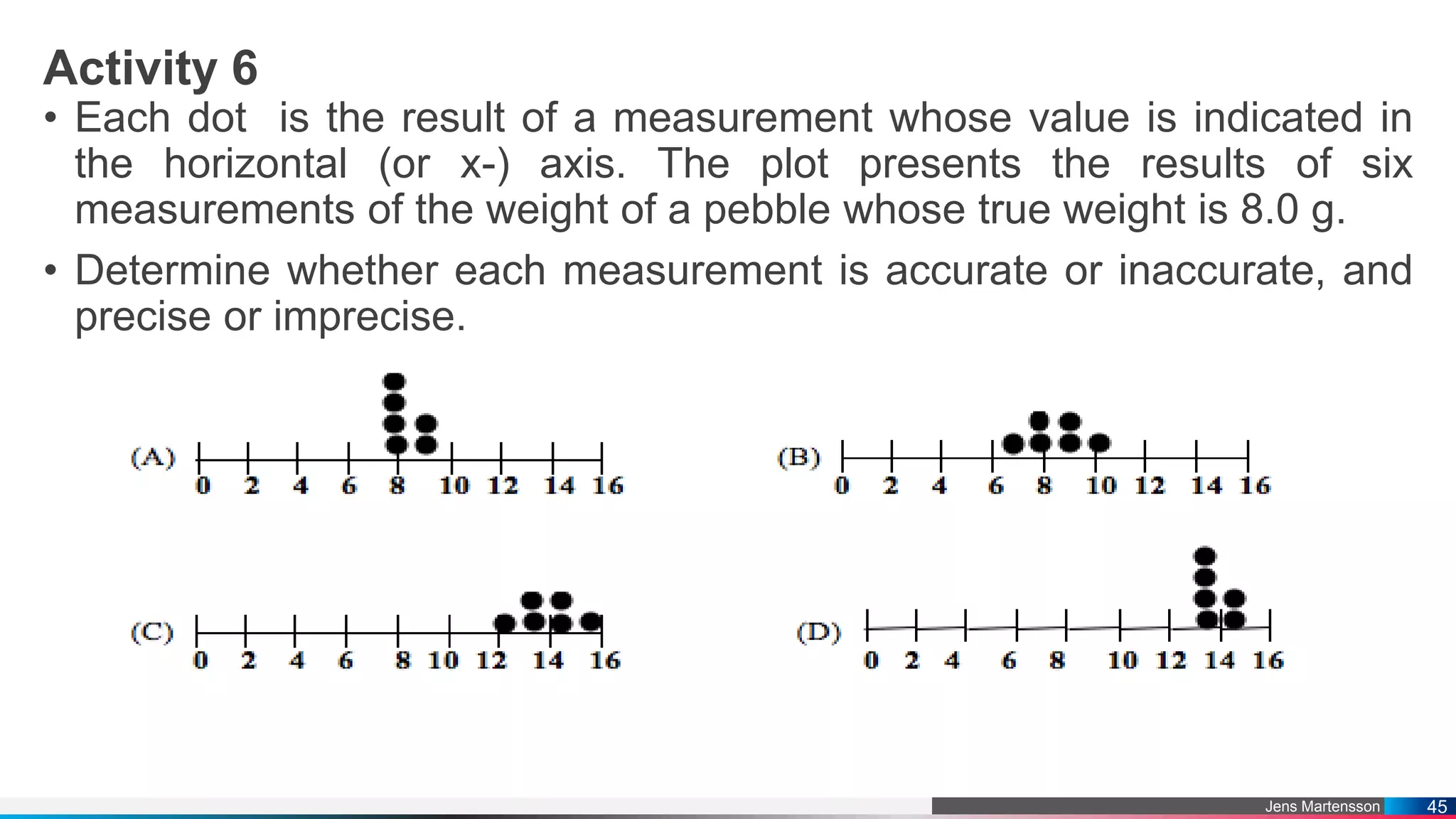
The document outlines a general chemistry curriculum for senior high school, covering topics such as the composition, structure, and properties of matter, alongside key concepts from physical and organic chemistry. It includes detailed lessons on matter properties, states of matter, measurements, and separation techniques, along with activities and practical applications. Additionally, it emphasizes the relevance of chemistry to everyday life and consumer products.