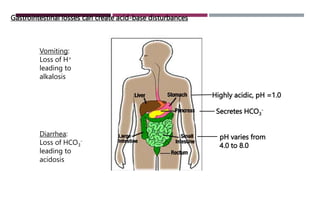
This document outlines a lecture on acid-base disorders given by Dr. Raneem AlSayed. The learning objectives are to recognize normal acid-base regulation and relationships, outline causes of respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis. The content will cover physiology of acid-base balance, case studies of different disorders, and the roles of lungs, kidneys, and buffer systems in regulation. Key concepts to be discussed are the pH scale, clinically significant acid-base pairs, respiratory versus renal regulation, and approaches to analyzing arterial blood gases.
























![Case 4
A 6 year old girl with severe gastroenteritis is admitted to the
hospital for fluid rehydration, and is noted to have a high [HCO3
-]
on hospital day #2. An ABG is ordered:
ABG: pH 7.47 Chem : Na+ 130
PCO2 46 K+ 3.2
HCO3
- 32 Cl- 86
PO2 96 HCO3
- 33
Urine pH: 5.8
Hypokalemic hypocholiremic metabolic alkalosis](https://image.slidesharecdn.com/acidbase-230829210202-9325cdb9/85/Acid-Base-Balance-30-320.jpg)