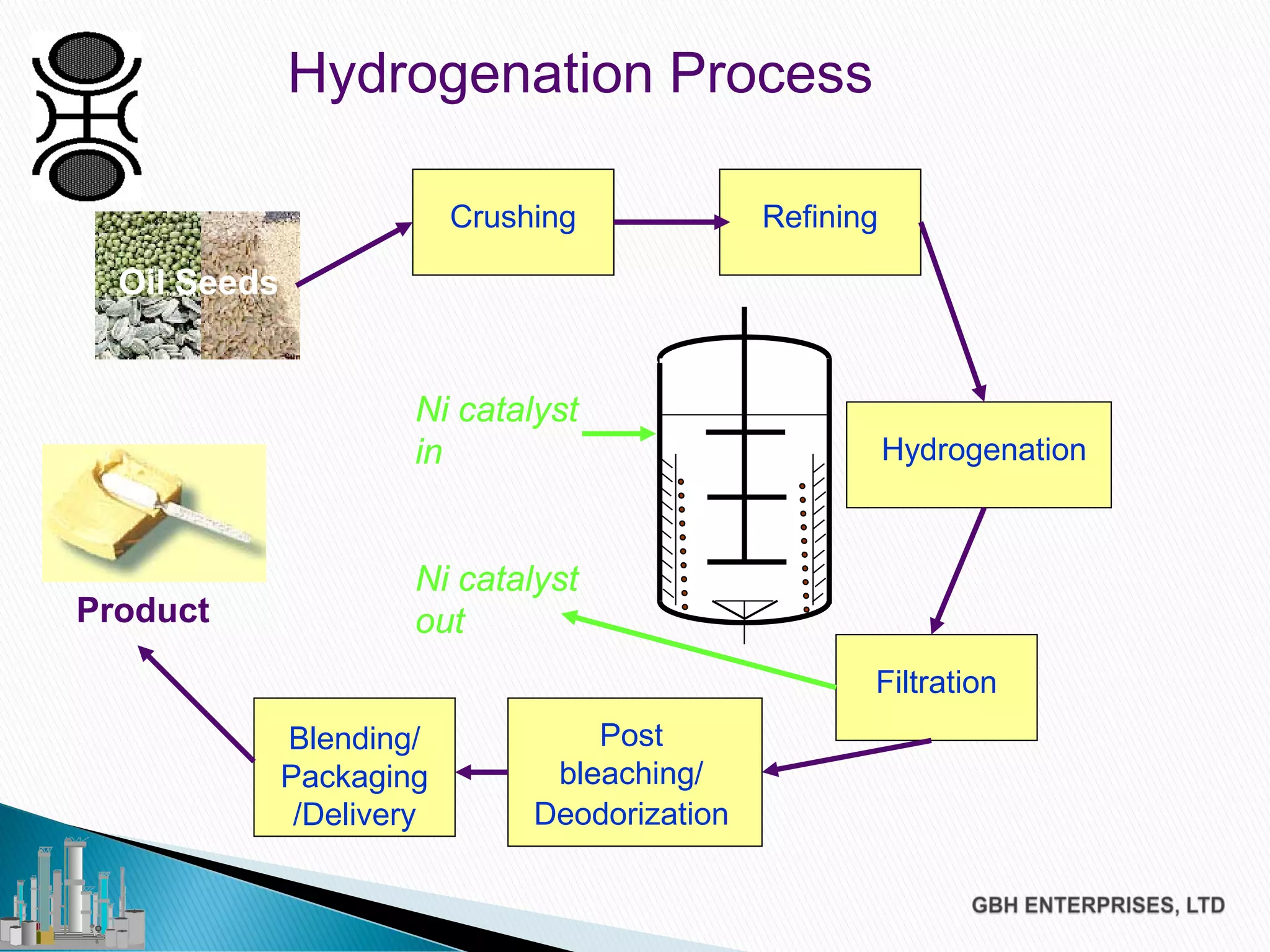
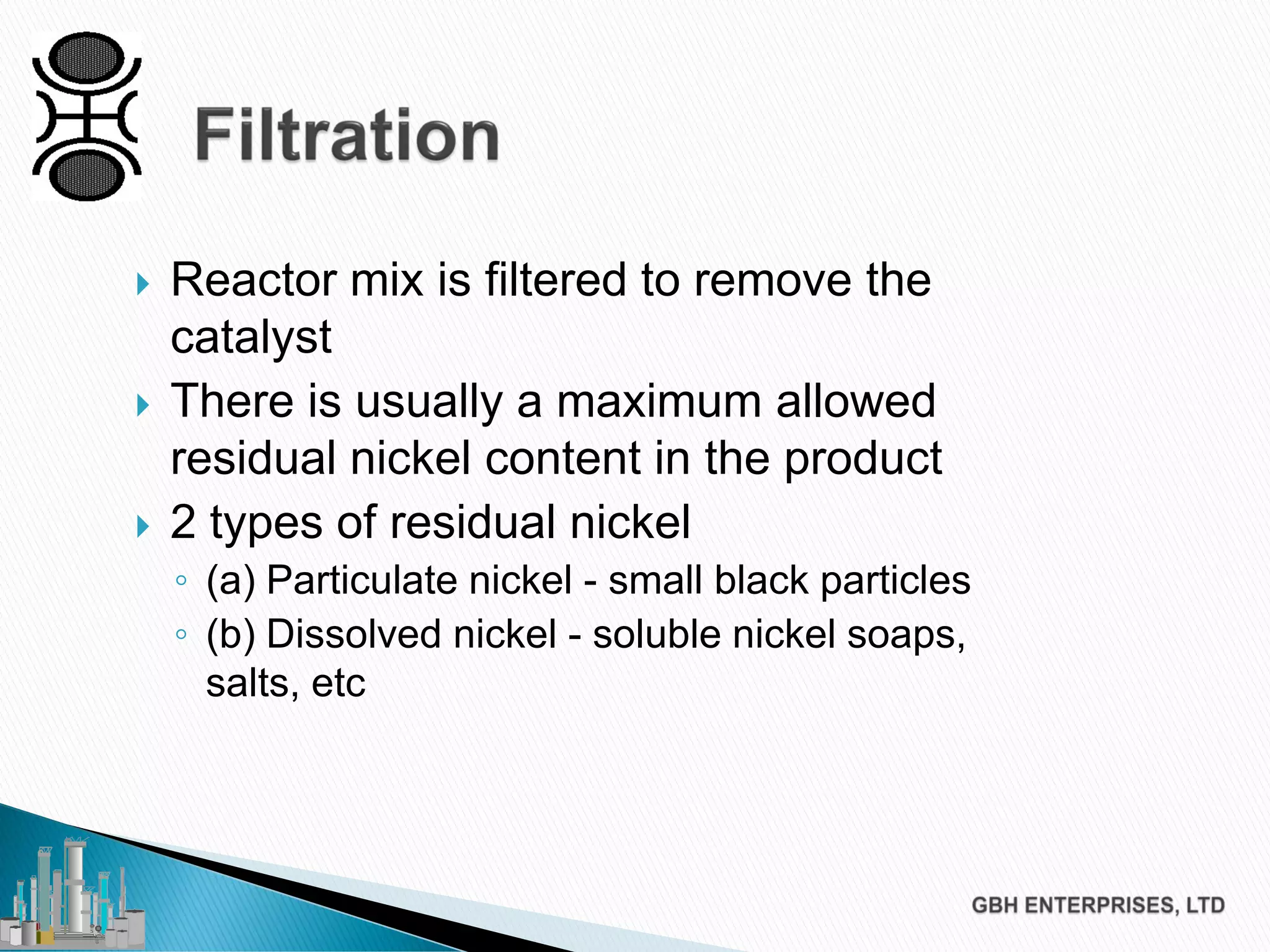
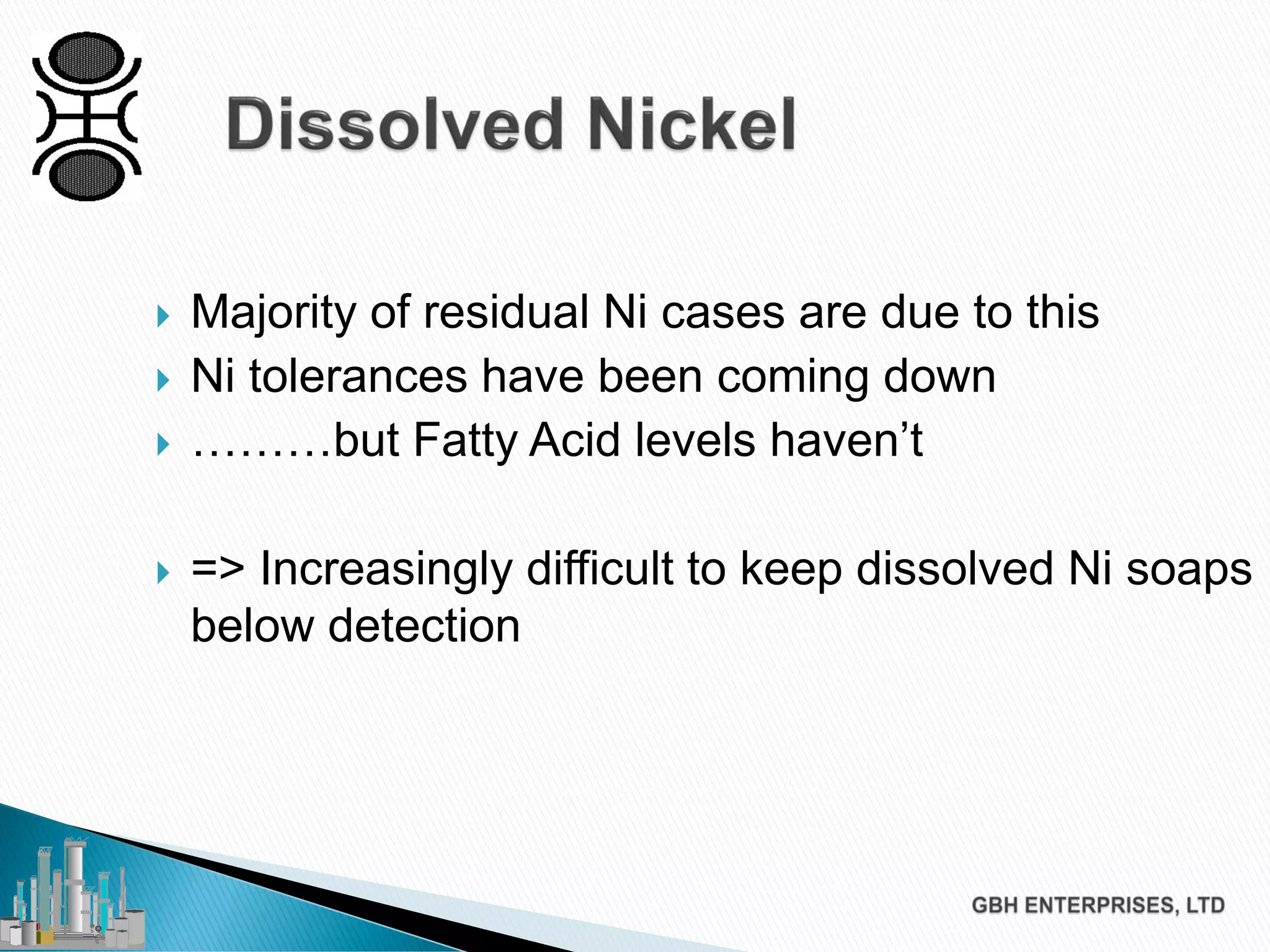
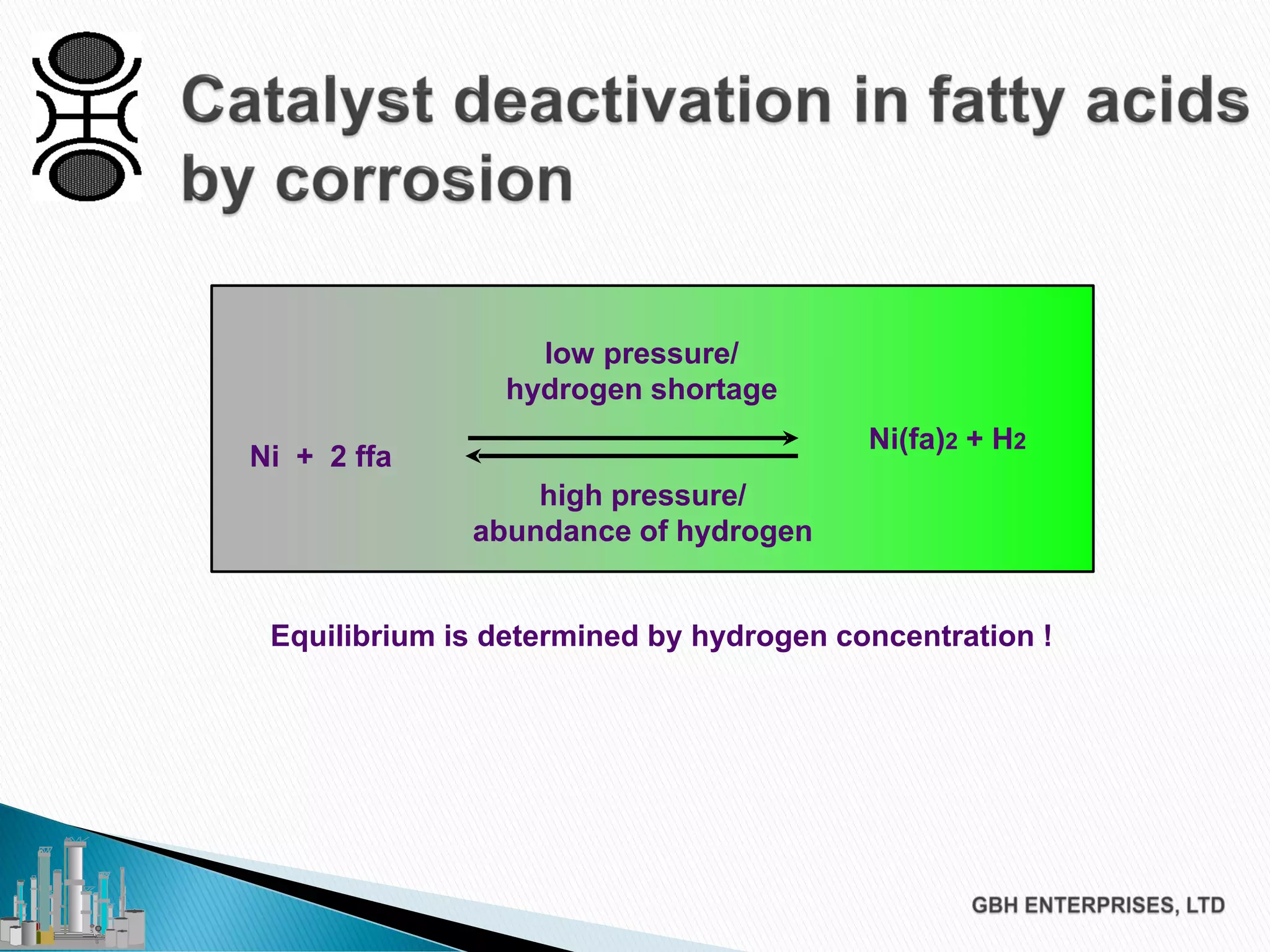
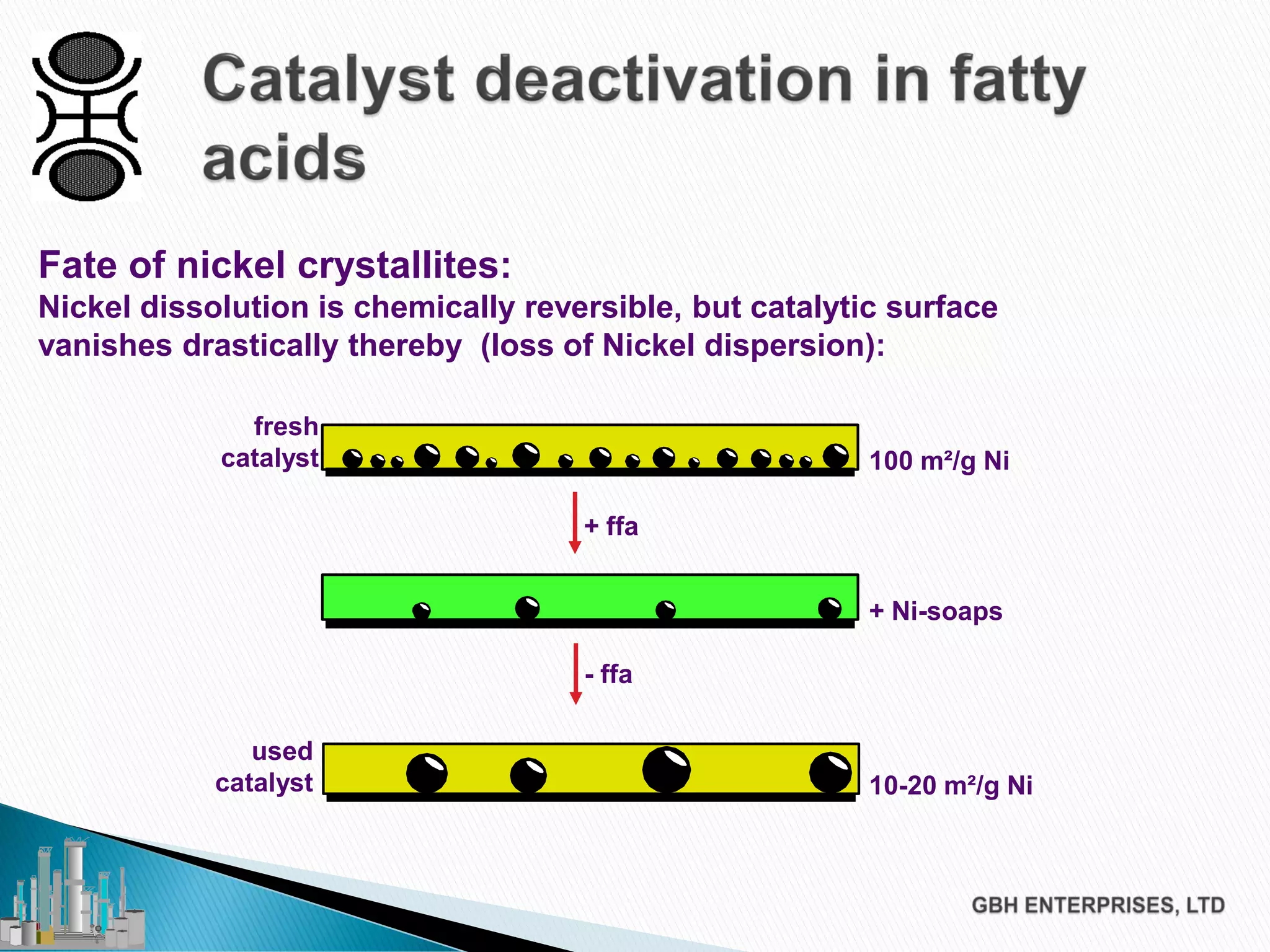
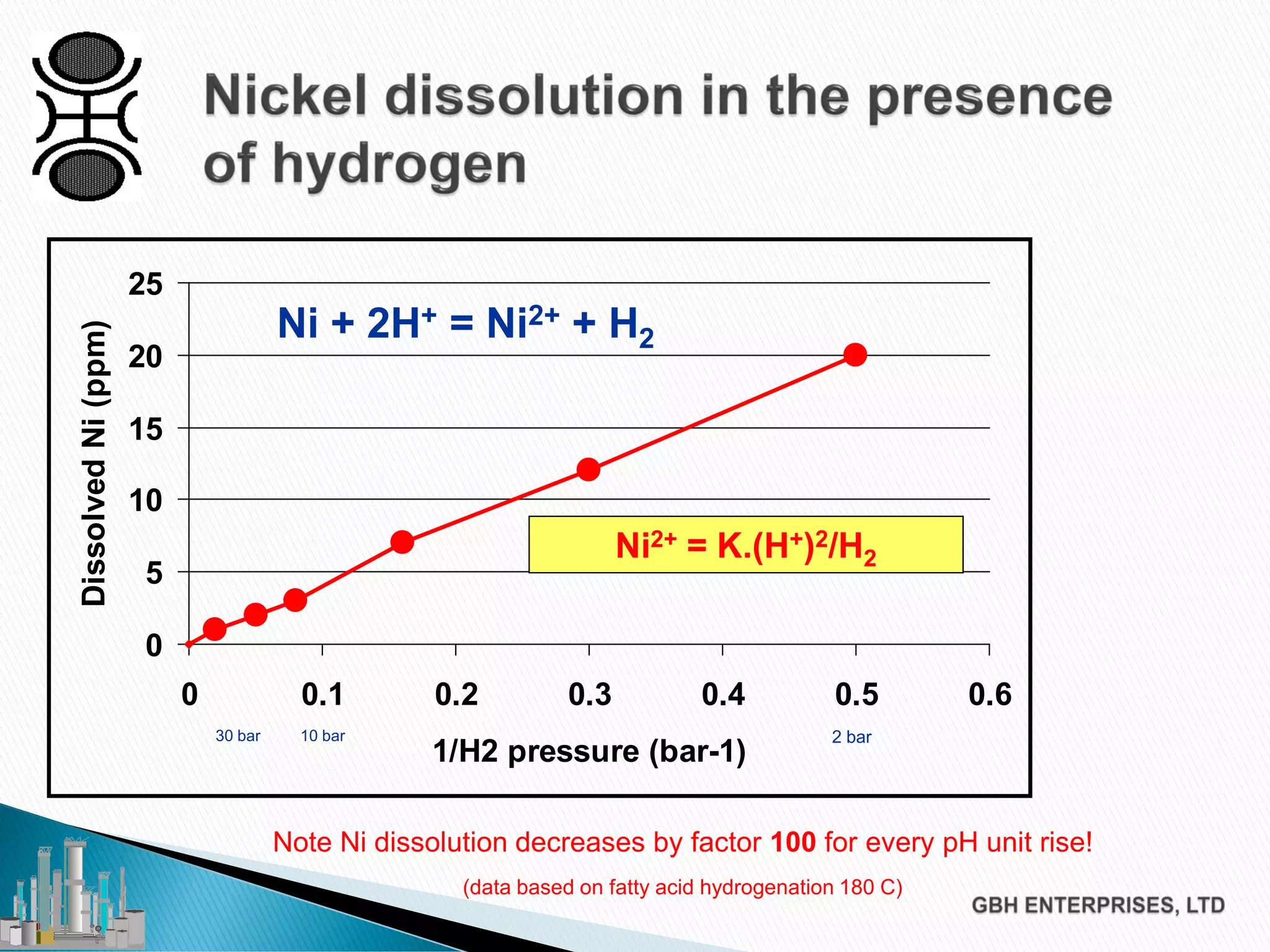
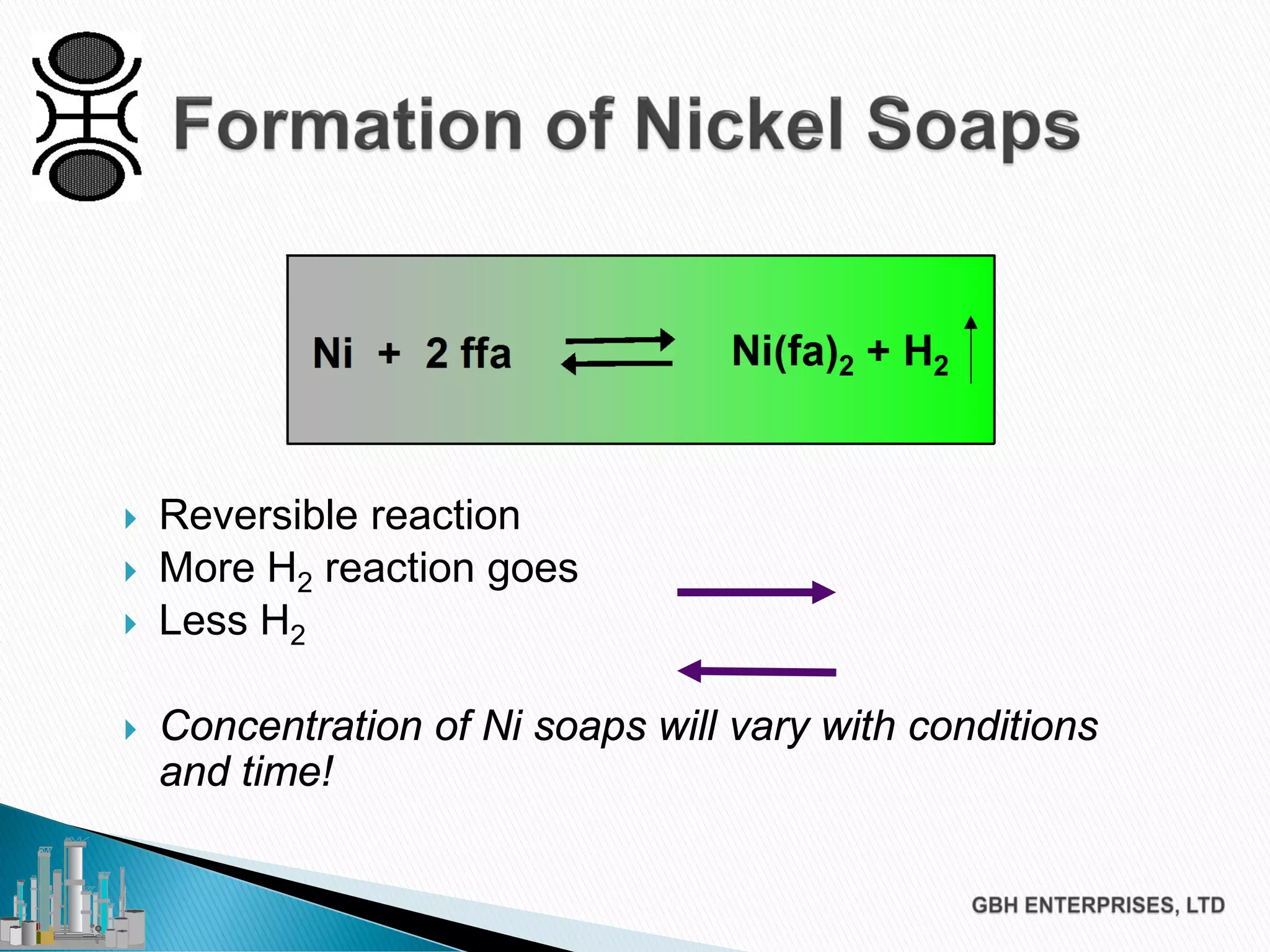
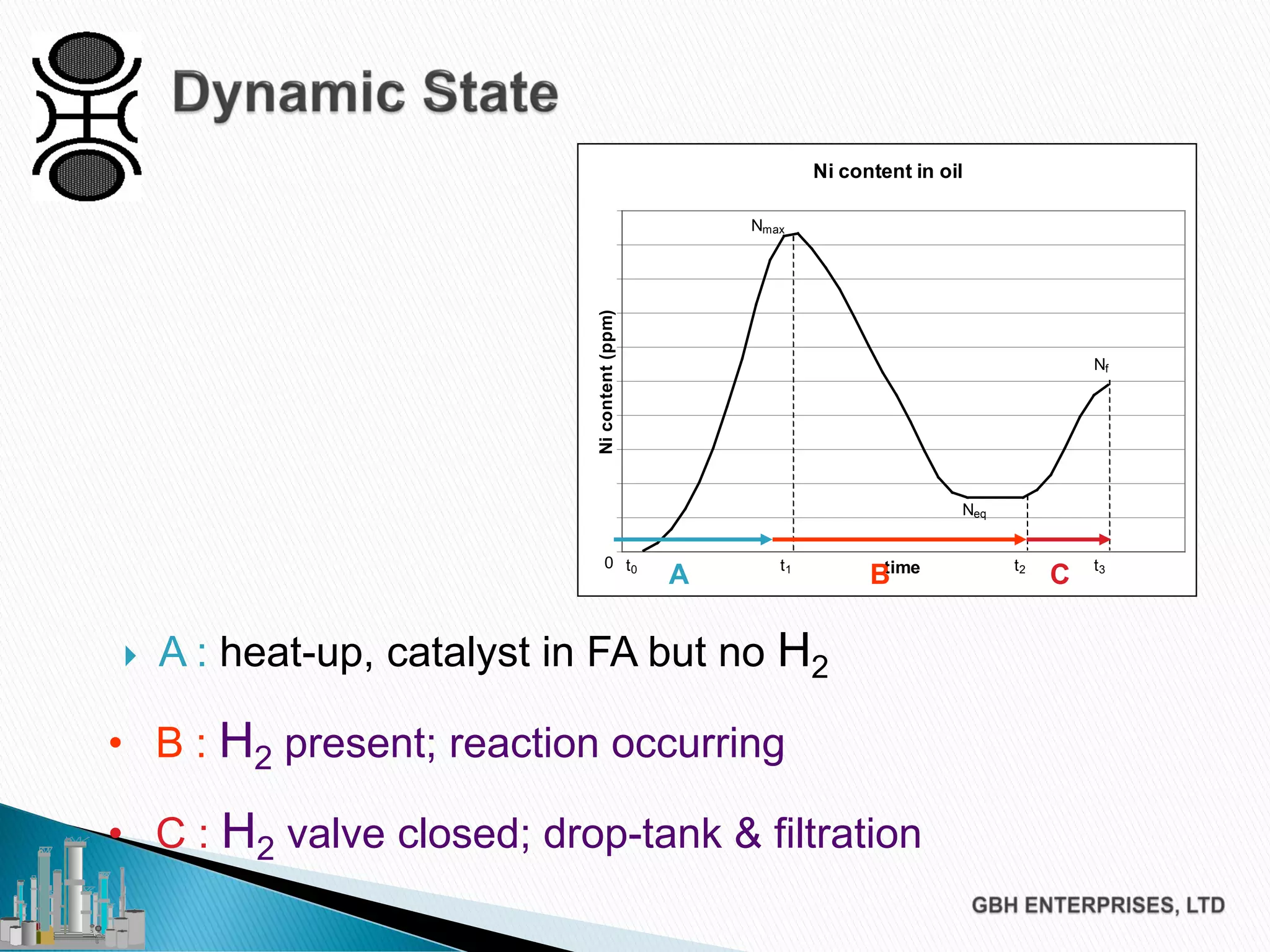
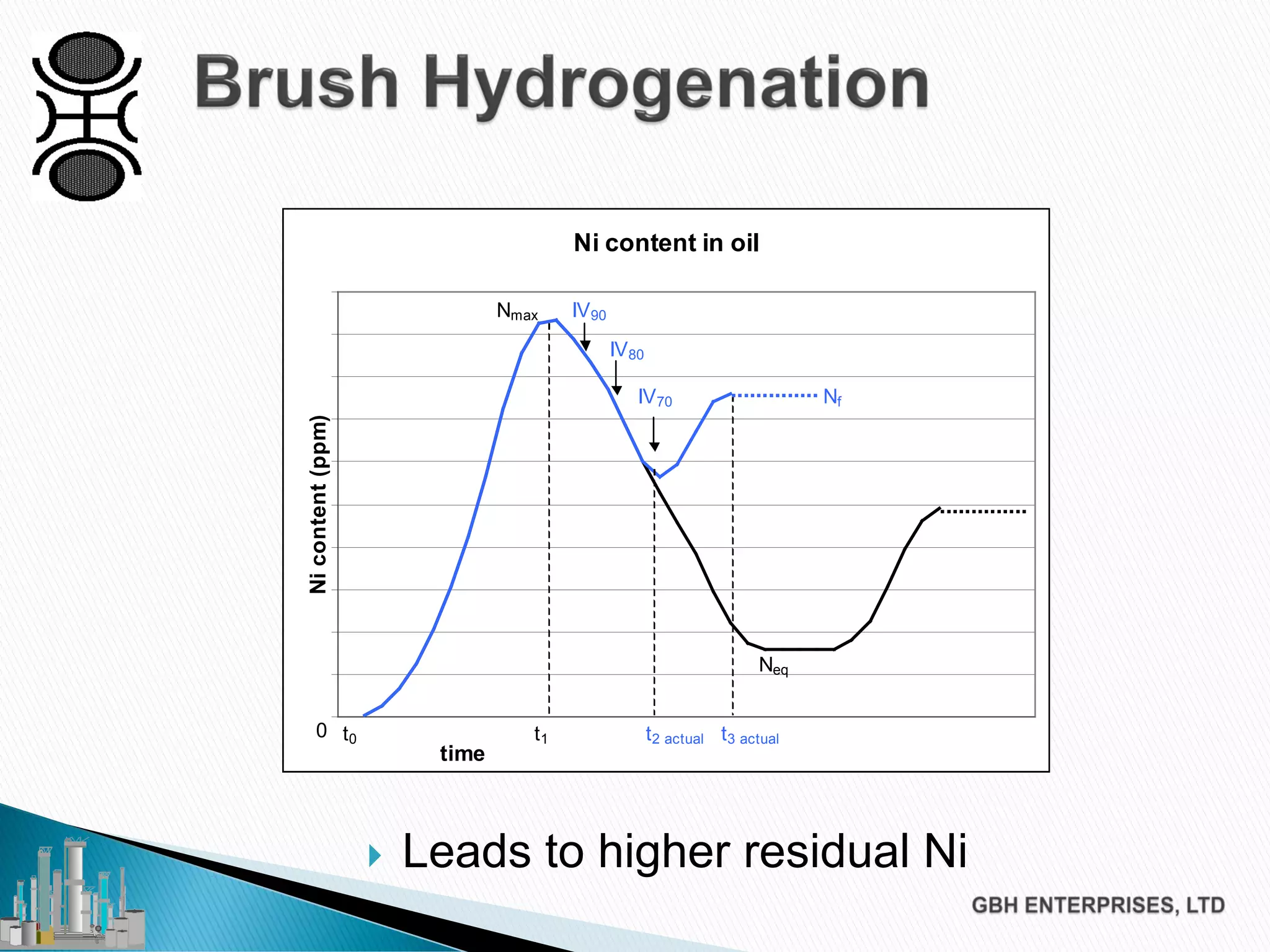
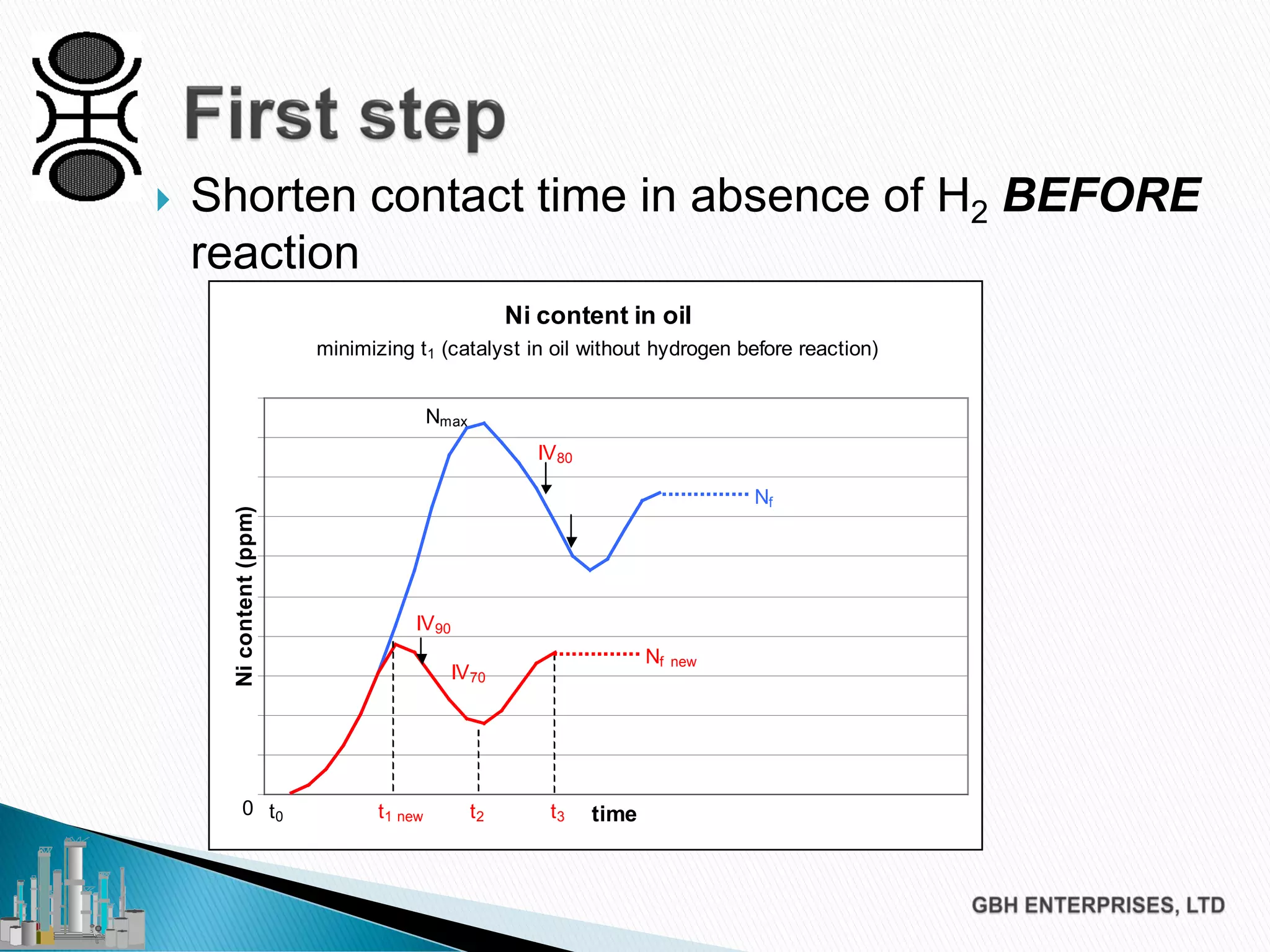
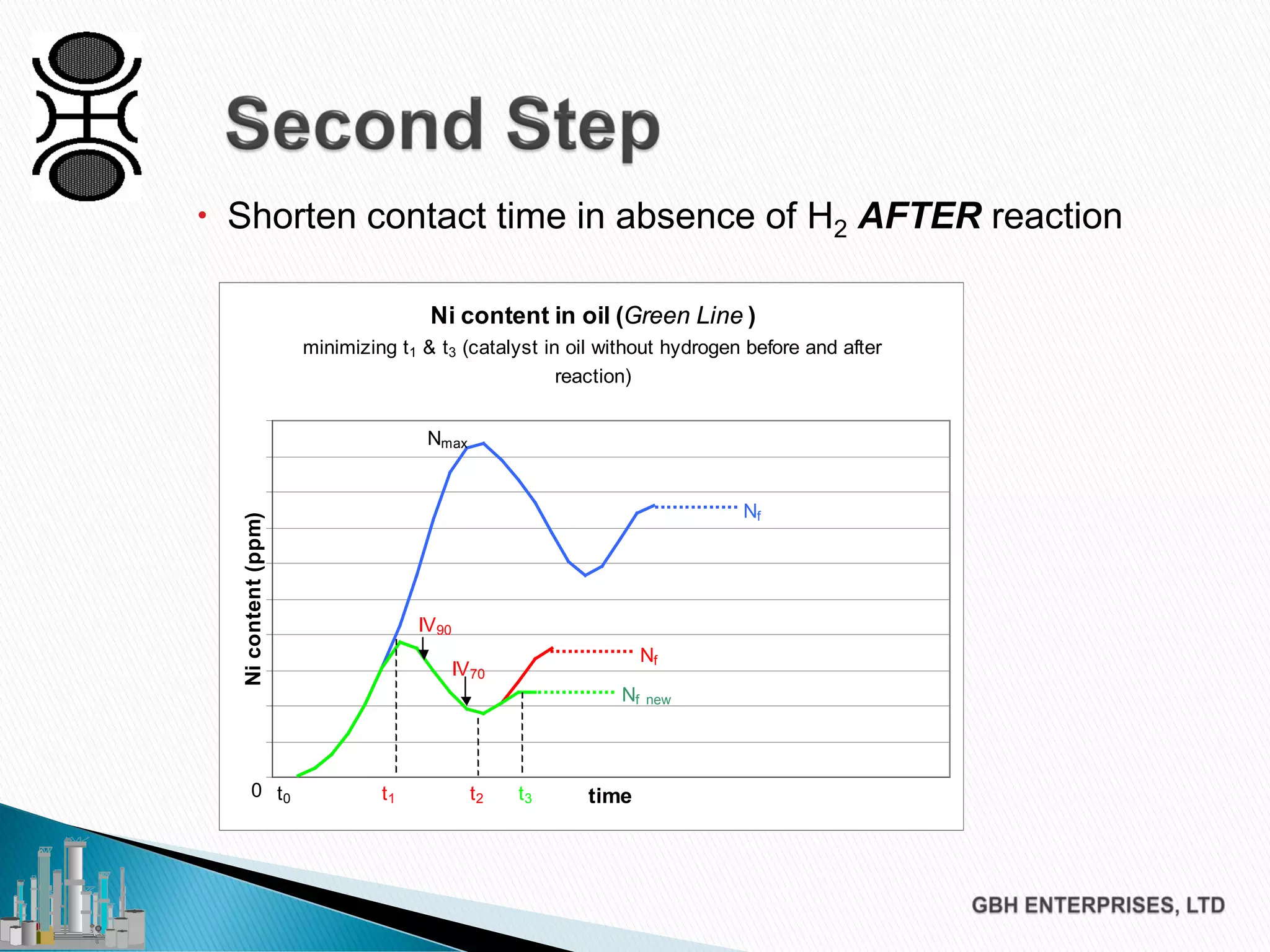
The document discusses the challenges of controlling residual nickel content in hydrogenation processes, focusing on dissolved and particulate nickel. It outlines strategies to minimize nickel soaps formation and improve filtration methods while highlighting the dynamic nature of nickel content based on processing conditions. Key recommendations include reducing free fatty acids (FFA), optimizing filtration temperature, and ensuring proper timing of catalyst addition relative to hydrogen presence.