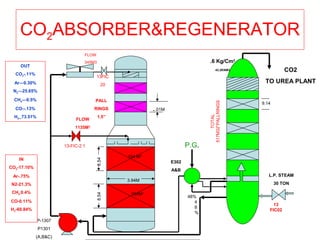
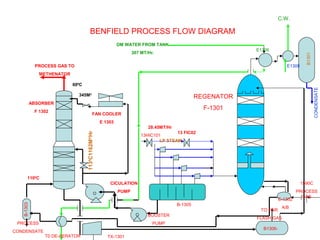
This document provides information on the Benfield process for removing carbon dioxide from gas streams. It discusses key aspects of the process including:
- Absorption of CO2 into a potassium carbonate solution and regeneration of the solution by heating.
- Use of an activator like DEA to improve CO2 absorption.
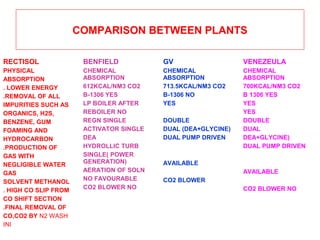
- Comparison with other CO2 removal processes like Rectisol and considerations for process selection.
- Parameters that affect the absorption and regeneration steps like pressure, temperature, and flow rates.
- Causes and prevention of corrosion in the system through vanadium addition and factors that can cause foaming of the solution.



![B. CHEMICAL ABSORPTION
BEST SUITED FOR LOW CO2 PARTIAL PRESSURE
1. MEA PROCESS:- MONO ETHANOL AMINE (REBOILER ENERGY IS
HIGH 2NH2(CH2)2 OH + CO2+H2O=[HO(CH2)2NH3]2CO3
DISADVANTAGE:-
(i)[HO(CH2)2NH3]2CO3+CO2+H2O= 2HO(CH2)2NH3HCO3
(ii) HO(CH2)2NH2+CO2= HO(CH2)2NHCOONH3(CH2)2OH
CARBOMATE IS CORROSSIVE IN HOTER PARTS OF MEA
• GV PROCESS
• CATACARB PROCESS
• BENFIELD PROCESS
C. PHYSIOCHEMICAL PROCESS
1. MDEA PROCESS( METHYLDIETHANOAMINE](https://image.slidesharecdn.com/benfieldsystem-150318091302-conversion-gate01/85/Benfield-system-6-320.jpg)