AQA GCSE Chemistry Summary Answers C8
•
0 likes•3,597 views
Answer for summary questions " C8 Rates & Equilibrium "
Report
Share
Report
Share
Download to read offline
Recommended
More Related Content
What's hot
What's hot (20)
IB Chemistry on Chemical Properties, Oxides and Chlorides of Period 3

IB Chemistry on Chemical Properties, Oxides and Chlorides of Period 3
Similar to AQA GCSE Chemistry Summary Answers C8
Similar to AQA GCSE Chemistry Summary Answers C8 (20)
67551071 au-gold cementation with zinc powder at low cyanide

67551071 au-gold cementation with zinc powder at low cyanide
Recently uploaded
This presentation was provided by William Mattingly of the Smithsonian Institution, during the fourth segment of the NISO training series "AI & Prompt Design." Session Four: Structured Data and Assistants, was held on April 25, 2024.Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"National Information Standards Organization (NISO)
APM Welcome
Tuesday 30 April 2024
APM North West Network Conference, Synergies Across Sectors
Presented by:
Professor Adam Boddison OBE, Chief Executive Officer, APM
Conference overview:
https://www.apm.org.uk/community/apm-north-west-branch-conference/
Content description:
APM welcome from CEO
The main conference objective was to promote the Project Management profession with interaction between project practitioners, APM Corporate members, current project management students, academia and all who have an interest in projects.APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
This presentation was provided by William Mattingly of the Smithsonian Institution, during the third segment of the NISO training series "AI & Prompt Design." Session Three: Beginning Conversations, was held on April 18, 2024.Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"National Information Standards Organization (NISO)
Mehran University Newsletter is a Quarterly Publication from Public Relations OfficeMehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Recently uploaded (20)
Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi

Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi
AQA GCSE Chemistry Summary Answers C8
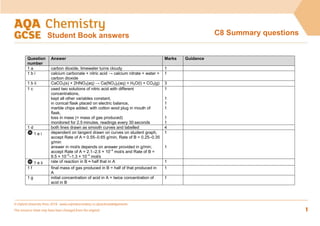
- 1. © Oxford University Press 2016 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original. 1 C8 Summary questions Student Book answers Question number Answer Marks Guidance 1 a carbon dioxide, limewater turns cloudy 1 1 b i calcium carbonate + nitric acid → calcium nitrate + water + carbon dioxide 1 1 b ii CaCO3(s) + 2HNO3(aq) → Ca(NO3)2(aq) + H2O(l) + CO2(g) 3 1 c used two solutions of nitric acid with different concentrations, kept all other variables constant, in conical flask placed on electric balance, marble chips added, with cotton wool plug in mouth of flask, loss in mass (= mass of gas produced) monitored for 2.5 minutes, readings every 30 seconds 1 1 1 1 1 1 1 d both lines drawn as smooth curves and labelled 4 1 e i dependent on tangent drawn on curves on student graph, accept Rate of A = 0.55–0.65 g/min, Rate of B = 0.25–0.35 g/min answer in mol/s depends on answer provided in g/min; accept Rate of A = 2.1–2.5 × 10−4 mol/s and Rate of B = 9.5 × 10−5 –1.3 × 10−4 mol/s 1 1 1 e ii rate of reaction in B ≈ half that in A 1 1 f final mass of gas produced in B = half of that produced in A 1 1 g initial concentration of acid in A = twice concentration of acid in B 1
- 2. © Oxford University Press 2016 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original. 2 C8 Summary questions Student Book answers Question number Answer Marks Guidance 1 h twice as many acid particles (H+(aq) ions) in A as in B in same volume of acid, so twice number of collisions in a given time between acid particles in solution and particles at surface of marble chips, so rate of reaction in A is twice rate in B 1 1 1 1 2 a kept temperature, concentration of acid, volume of acid, mass of zinc constant, only varied surface area of zinc in each test 2 1 2 b hydrogen, lighted splint pops 1 1 2 c Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) 2 2 d line 1 1 2 e largest pieces 1 2 f steepest line, rising to same level as other three, levelling off first 1 1 2 g The smaller the pieces of zinc, the larger its surface area and the more zinc particles exposed to react with acid particles moving randomly in solution. Therefore, frequency of collision between reactant particles increases, causing an increase in rate of reaction. 1 1 1 GCSE Chemistry only 2 h 48 cm3 or 0.048 dm3 3
- 3. © Oxford University Press 2016 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original. 3 C8 Summary questions Student Book answers Question number Answer Marks Guidance 3 a substance that increases rate of a reaction but remains chemically unchanged at end of reaction 1 1 3 b 2H2O2(aq) → 2H2O(l) + O2(g) 3 3 c Measure time to collect a fixed volume of oxygen using same mass of catalyst with same surface area in same volume and concentration of hydrogen peroxide solution at same temperature in each test. 1 1 1 1 1 GCSE Chemistry only 3 d 2.5 cm3 /s = 1.0 × 10−4 mol/s 3 (answer to 2 sig. fig. consistent with data provided) 3 e Filter off insoluble metal oxides after reaction, wash with distilled water and dry in a warm oven / leave to dry / dab with filter paper to dry. 1 1 1