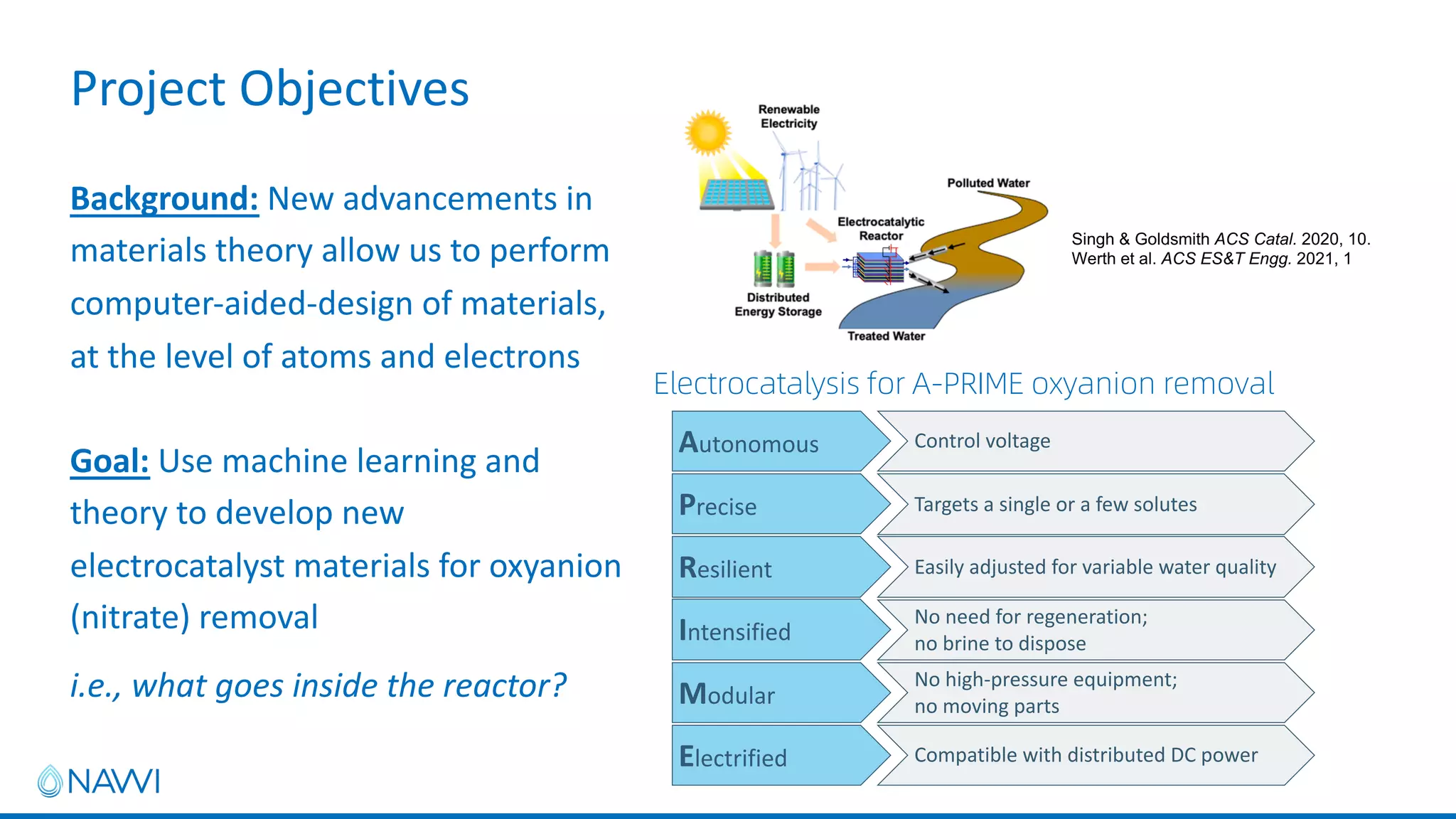
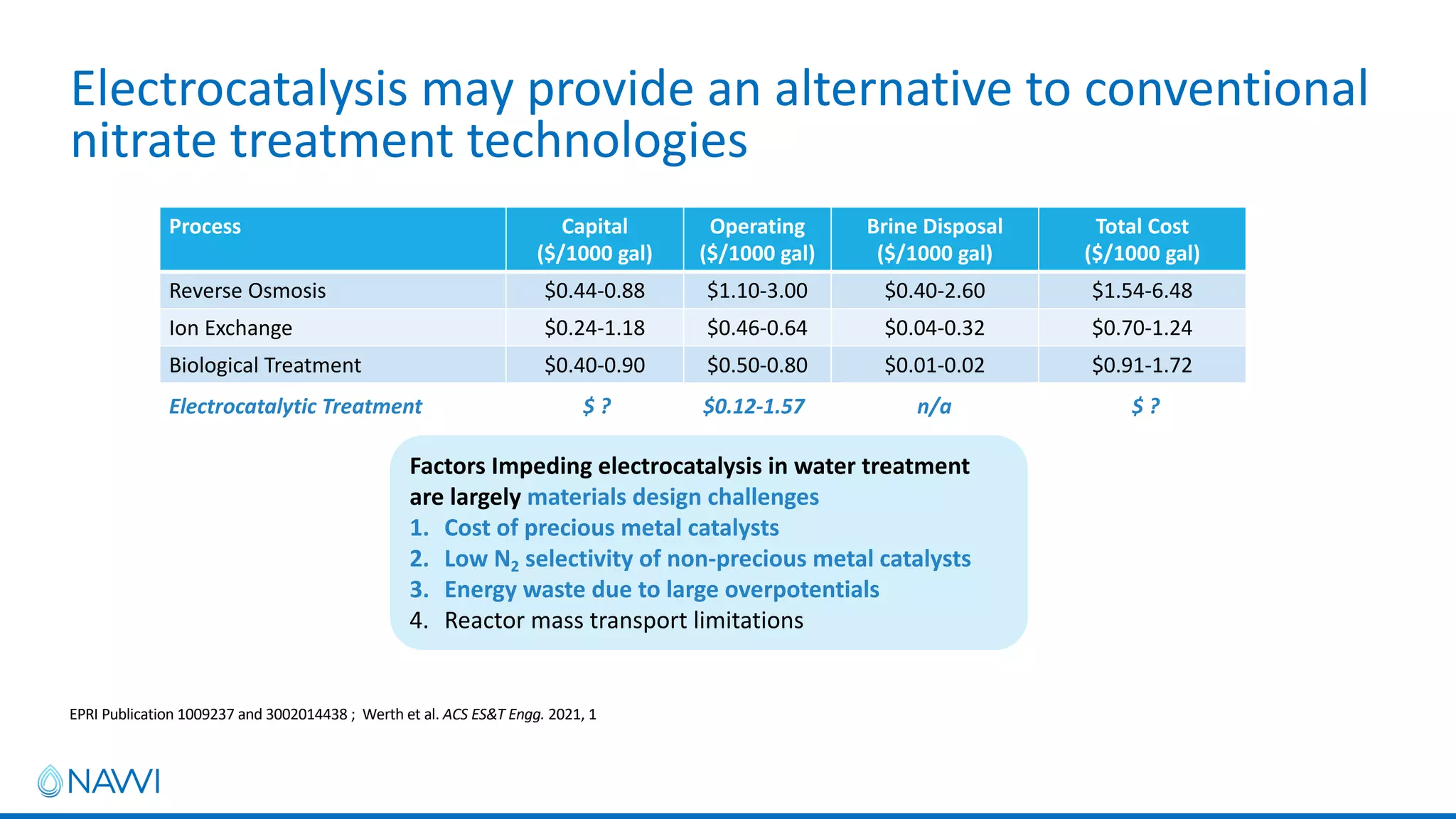
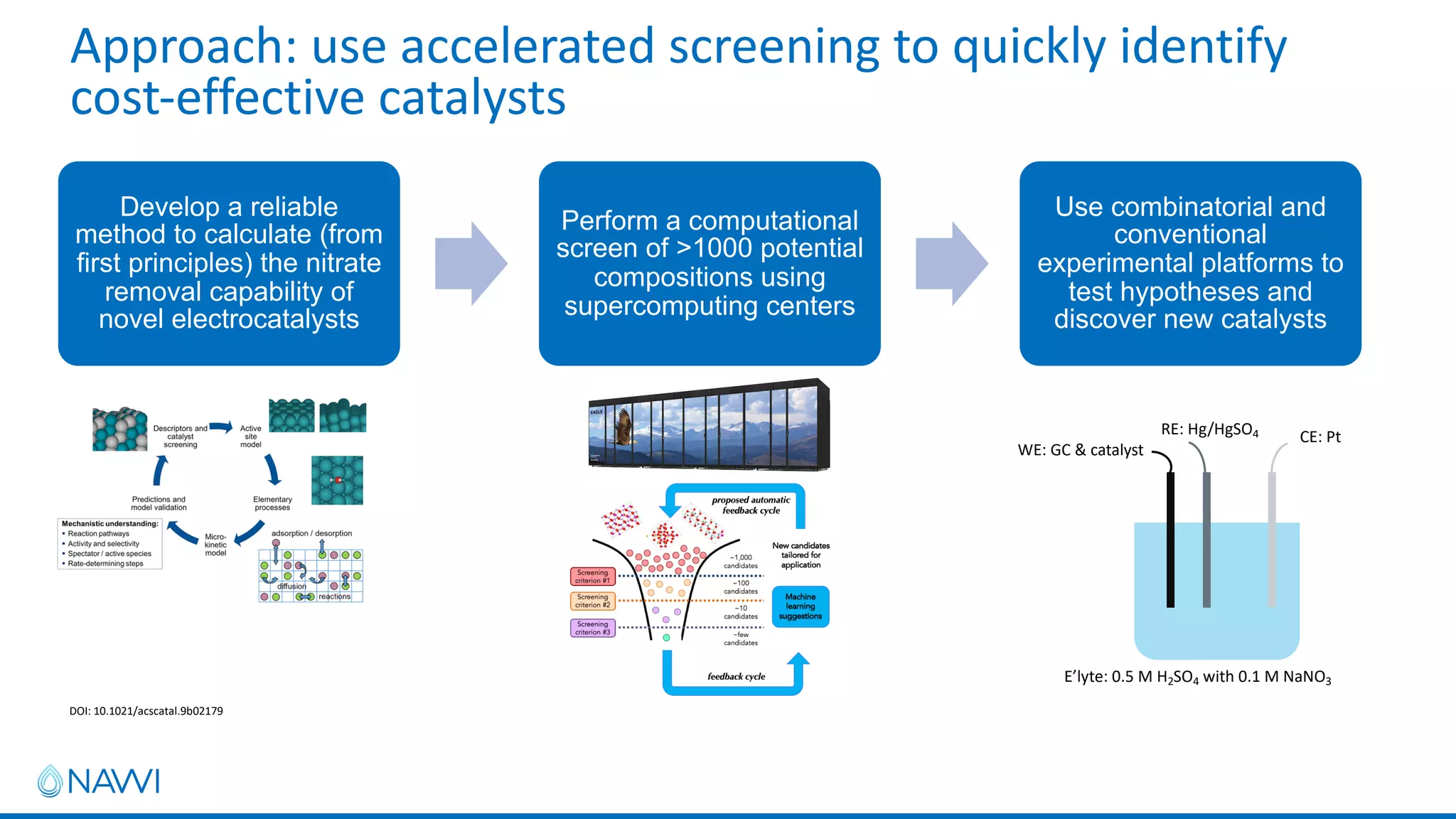
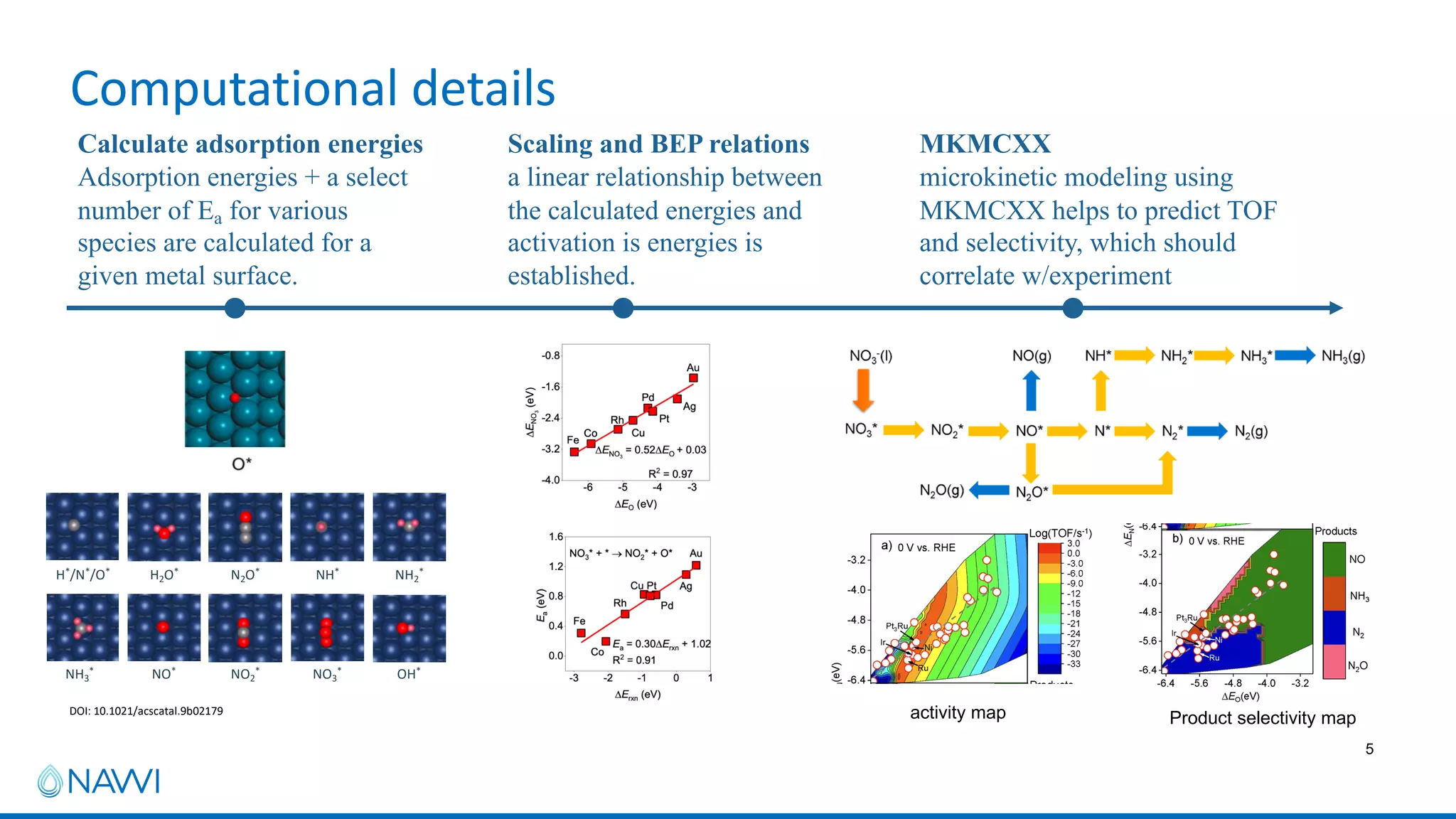
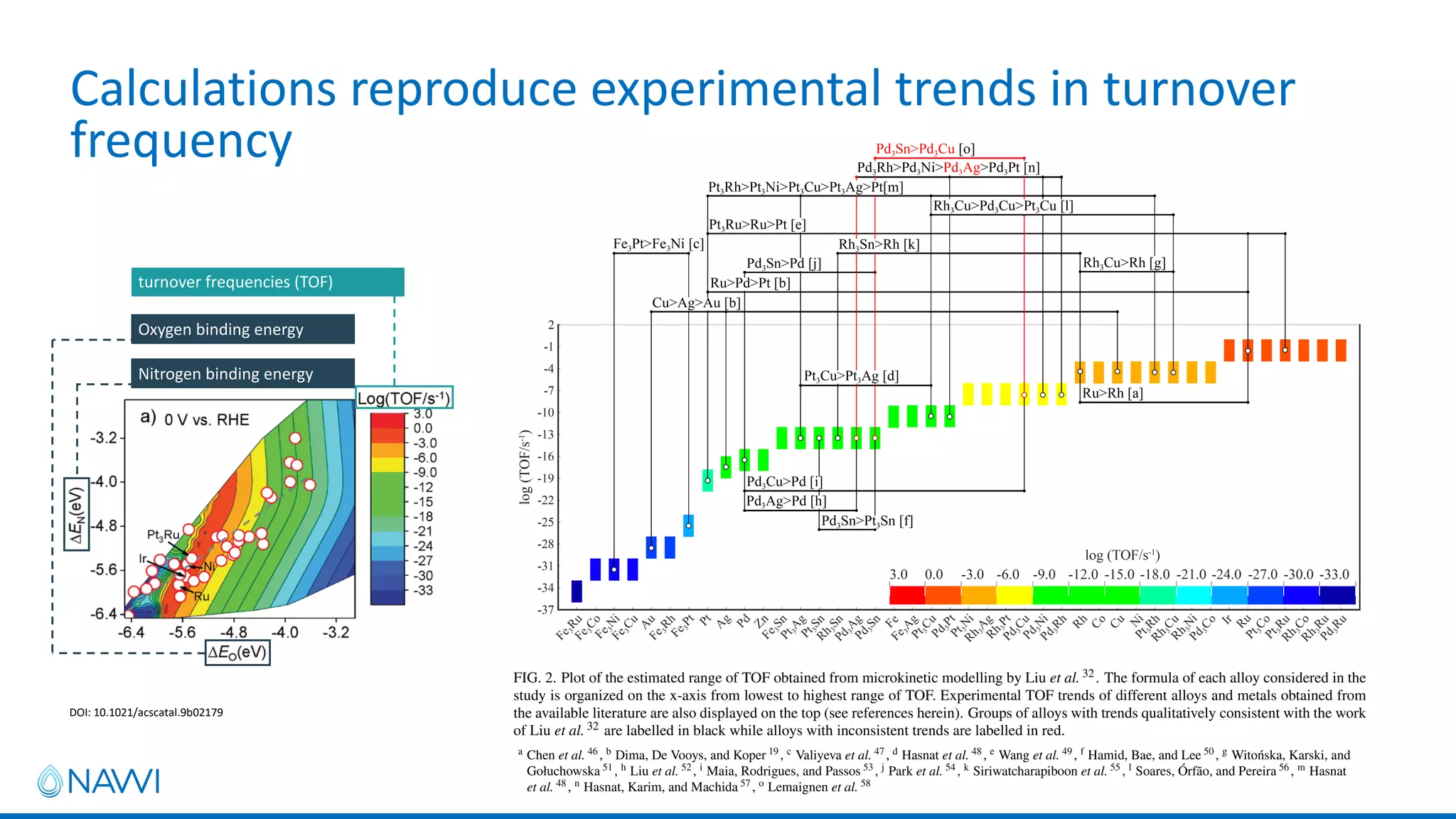
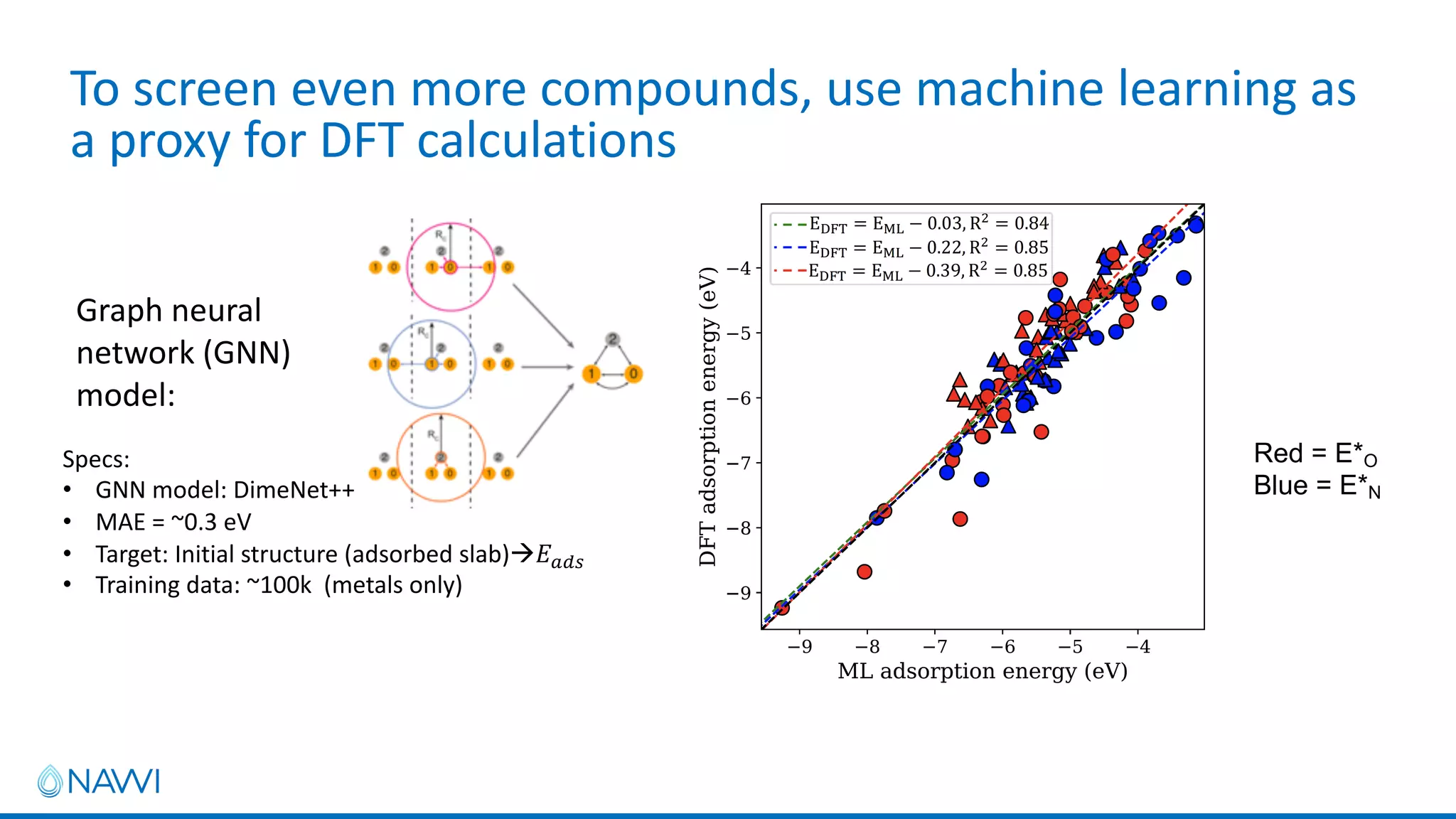
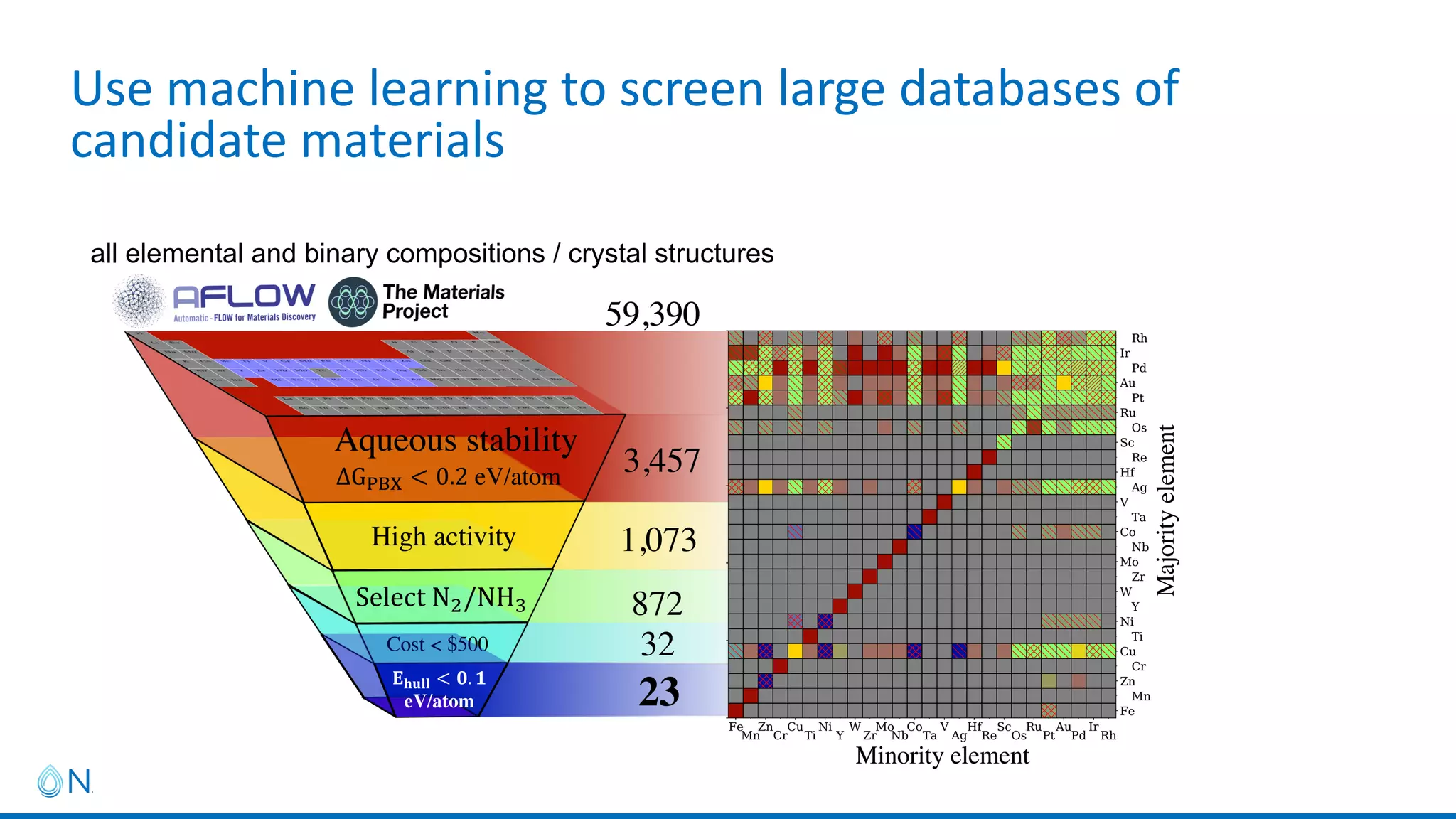
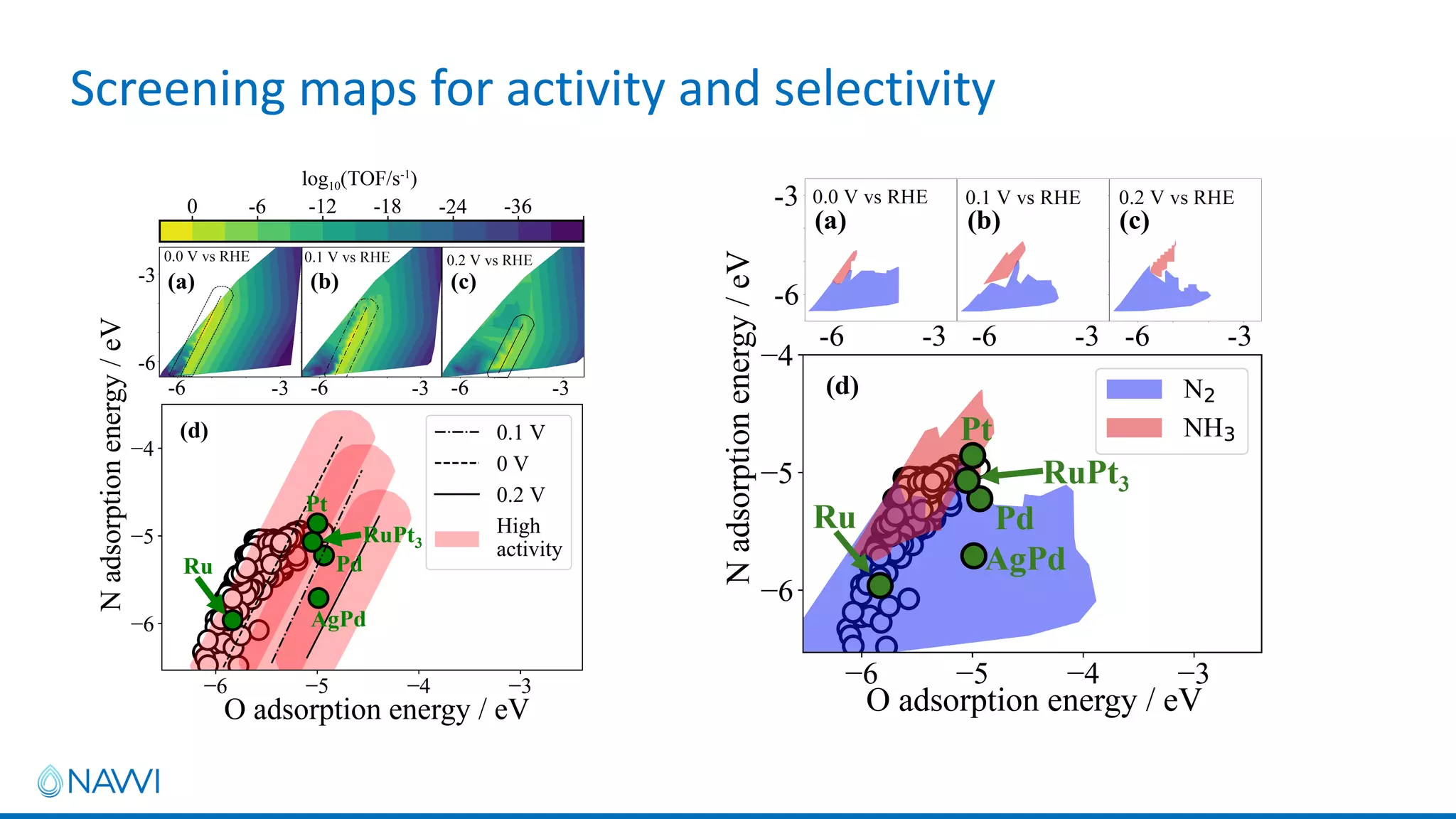
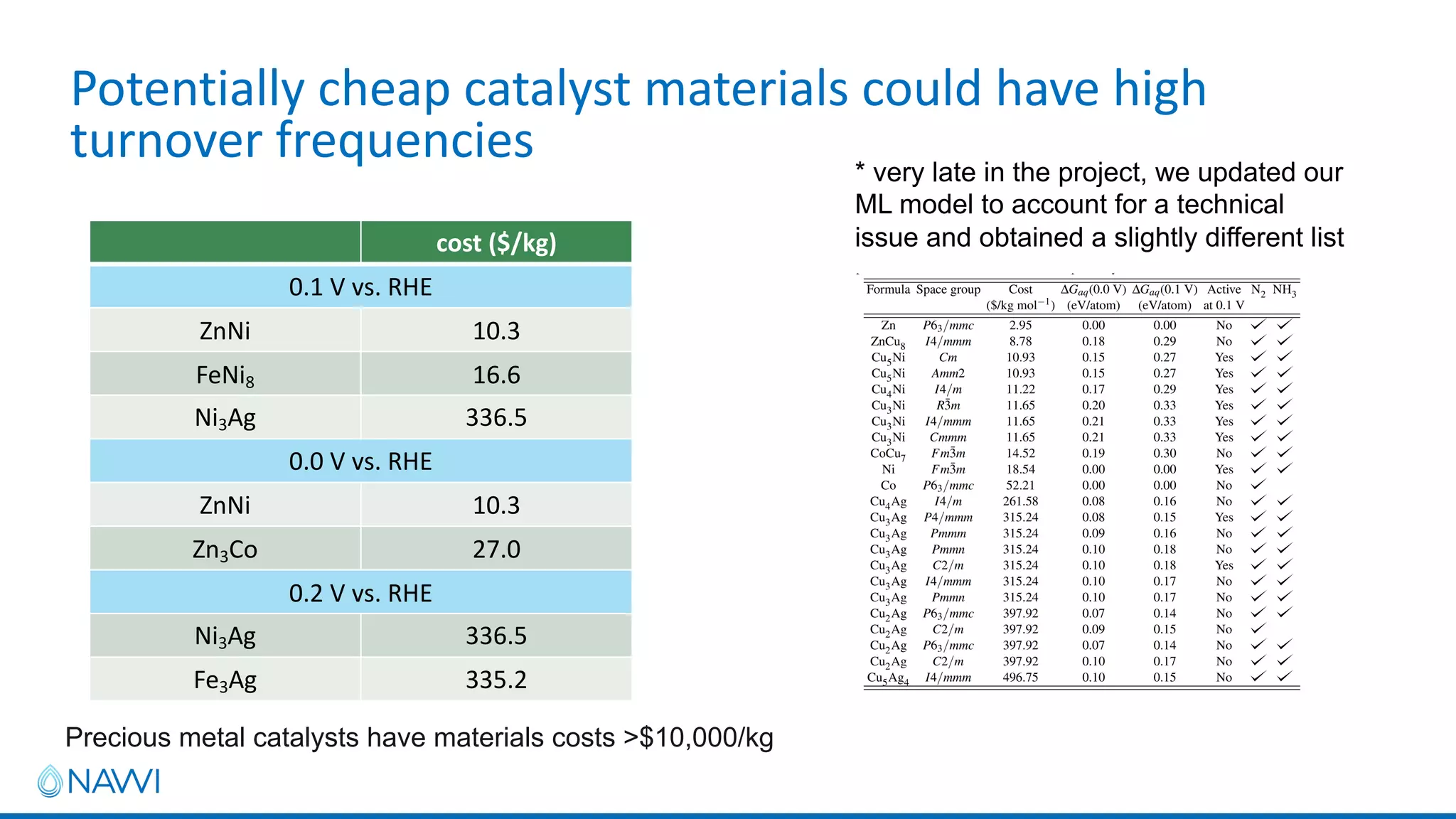
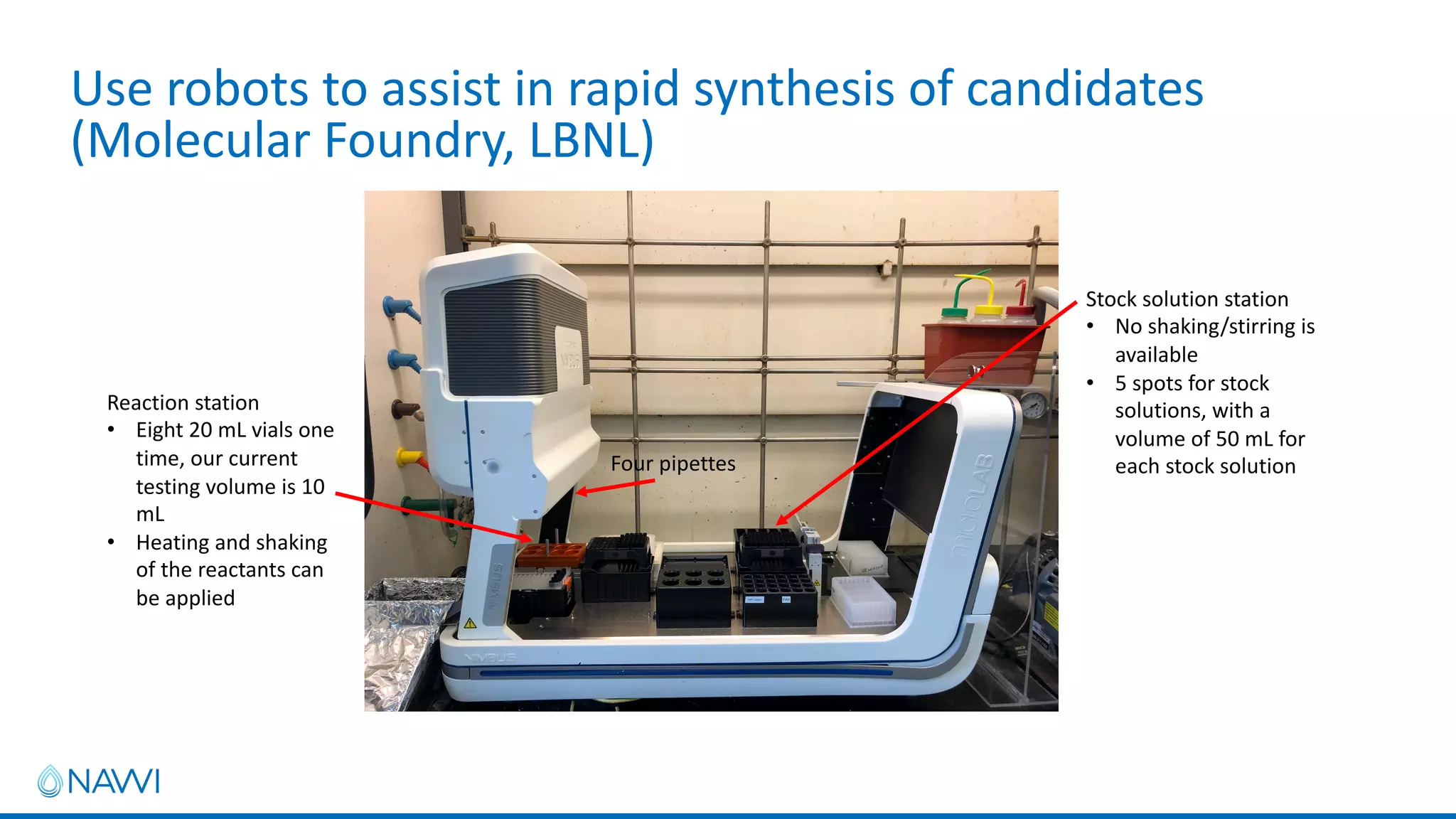
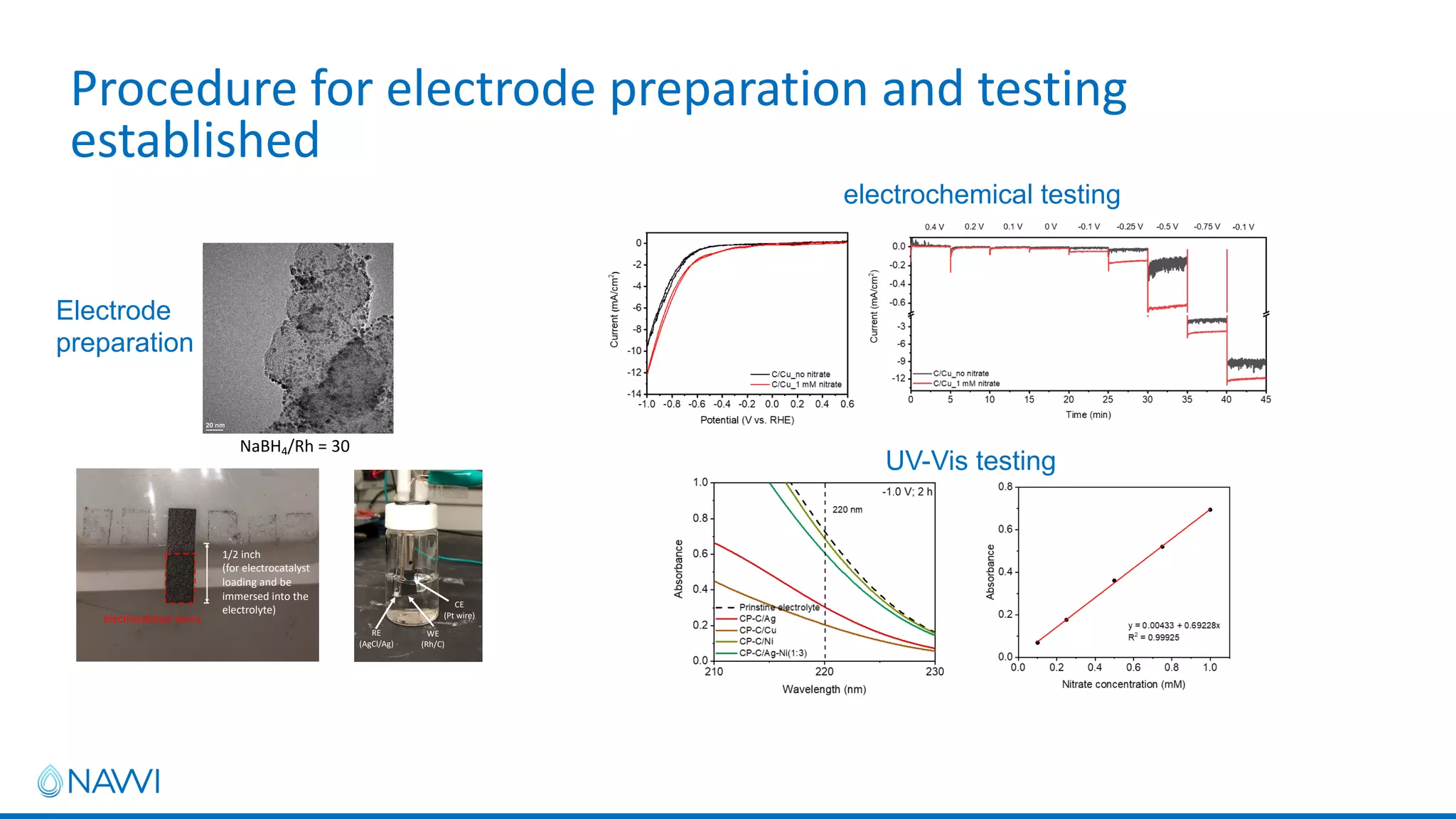
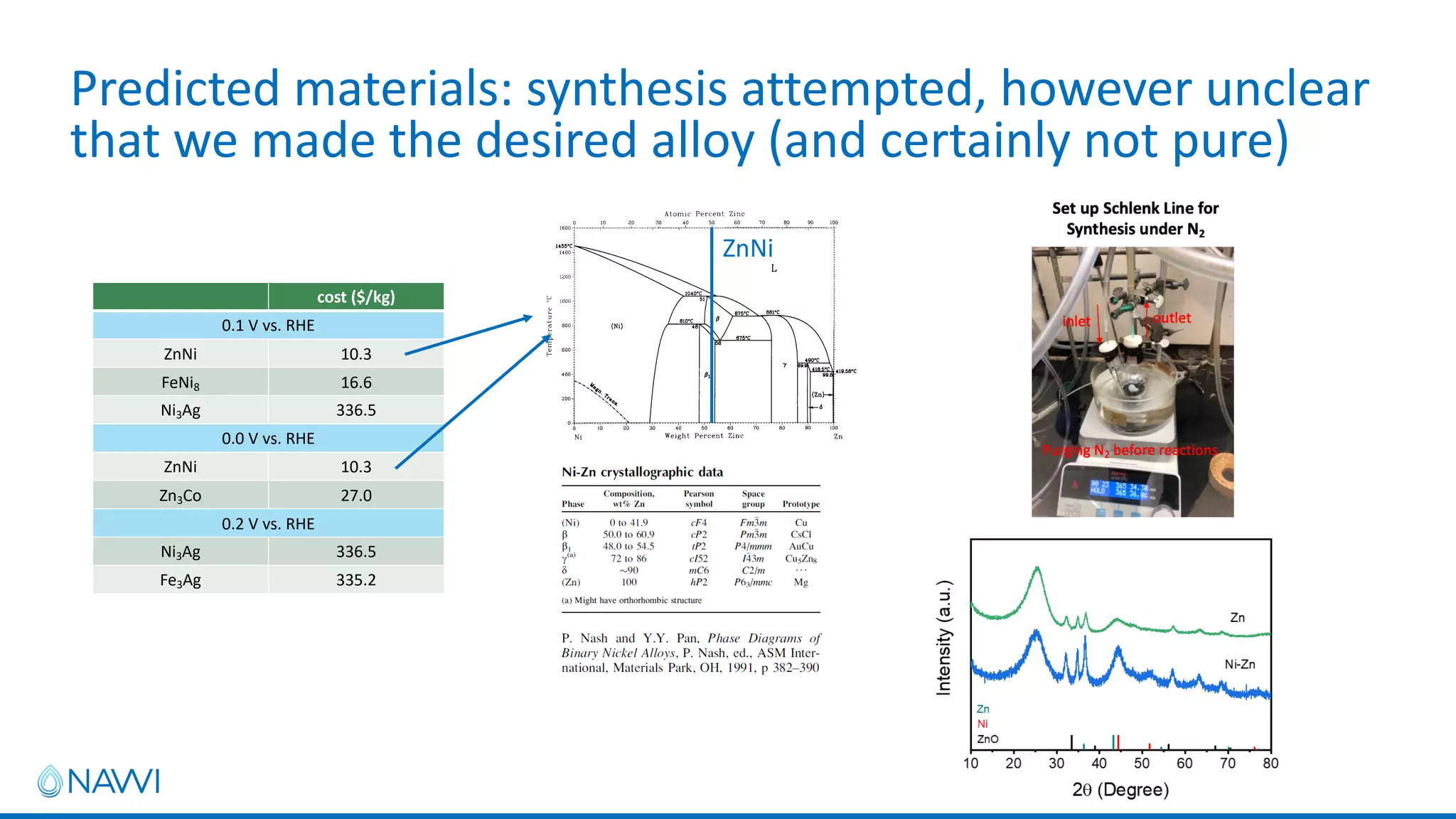
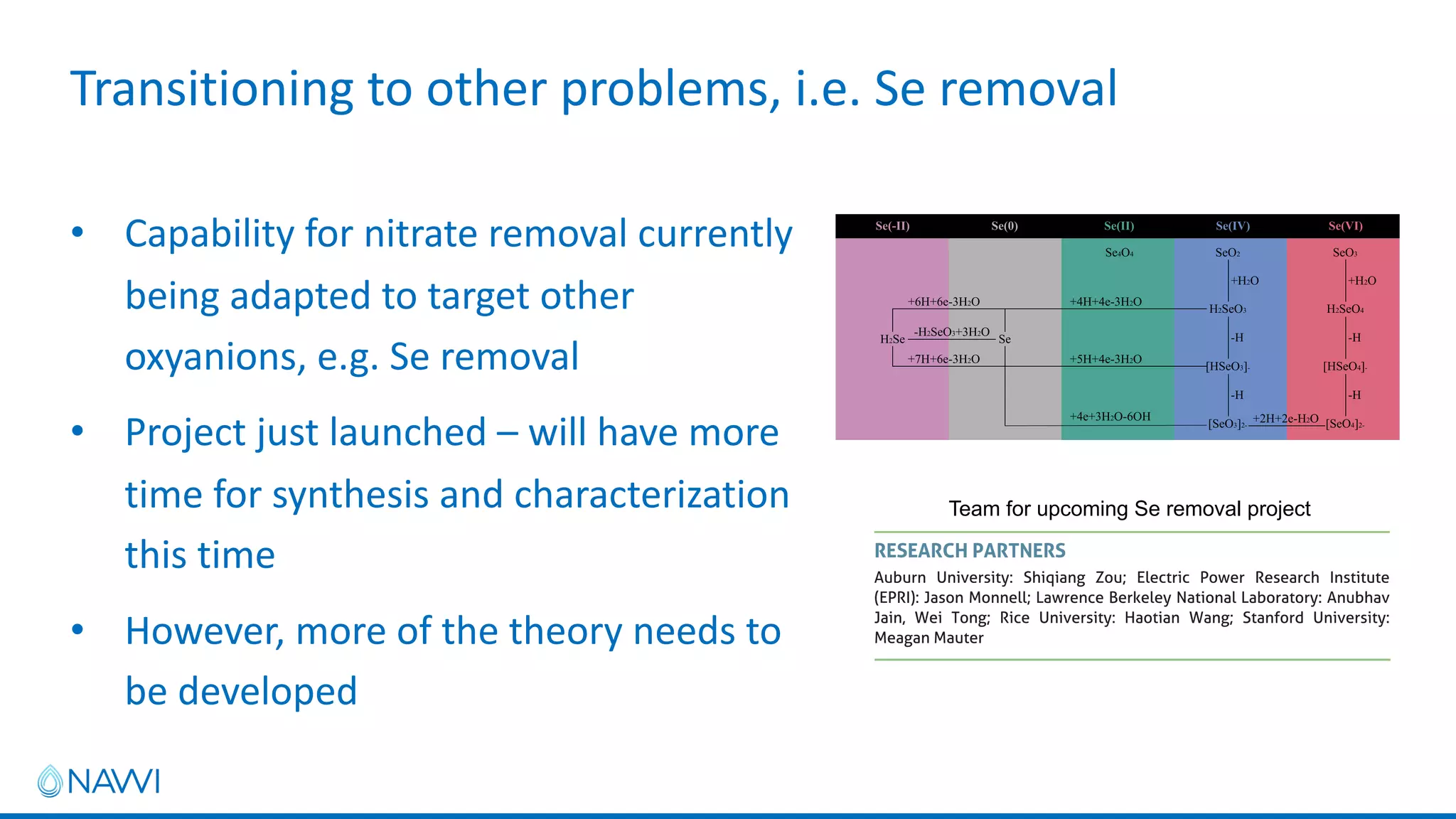
This project aims to develop new electrocatalyst materials for nitrate removal from water using machine learning and computational screening. The team performed calculations on over 1,000 potential compositions to identify promising catalysts with low costs. Experimental synthesis of candidates such as ZnNi and Zn3Co was attempted but it is unclear if the desired alloys were produced. The screening approach is now being applied to identify materials for selenium removal. If successful, low-cost catalysts could be developed to reduce the costs of electrocatalytic water treatment.