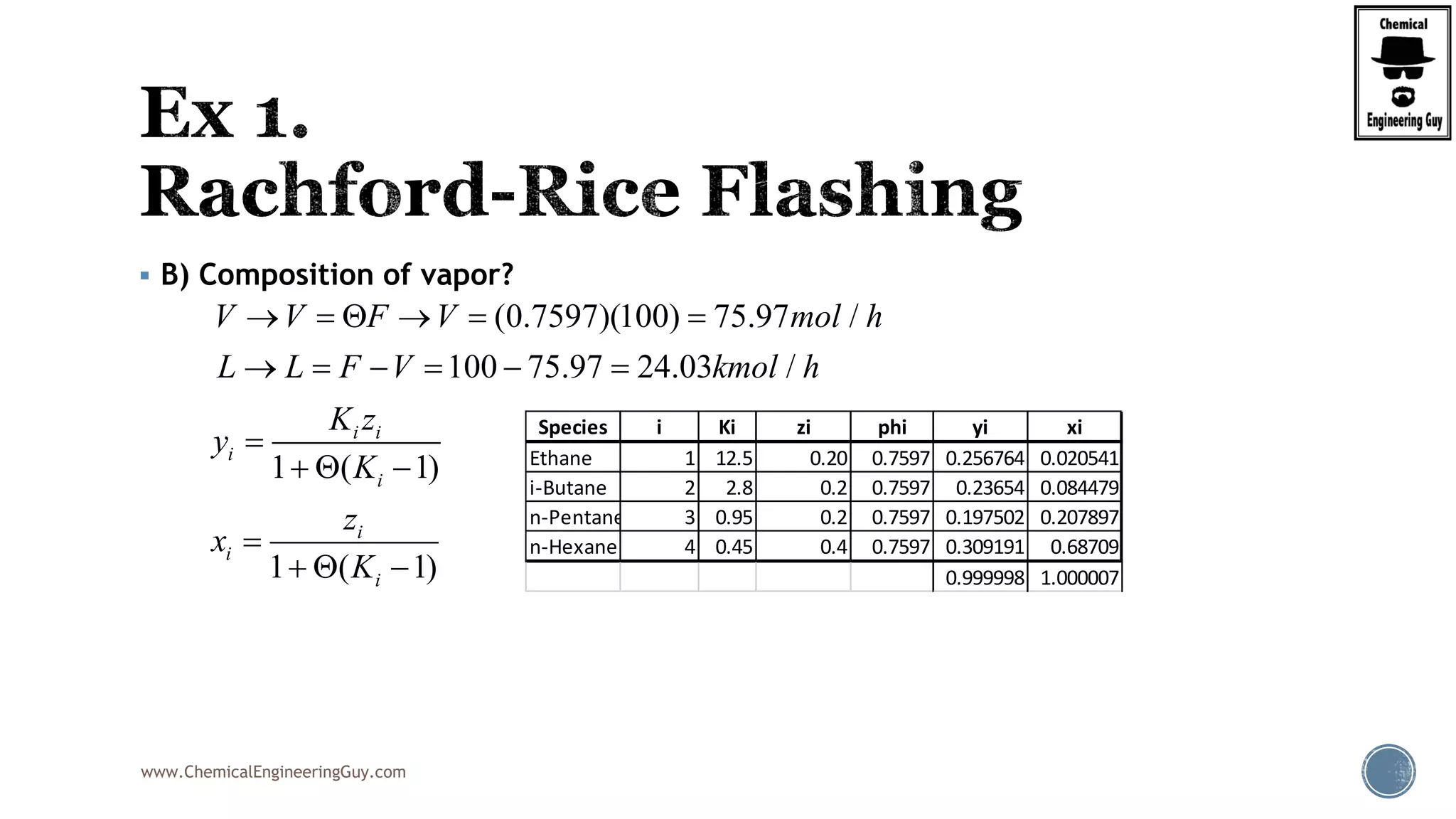
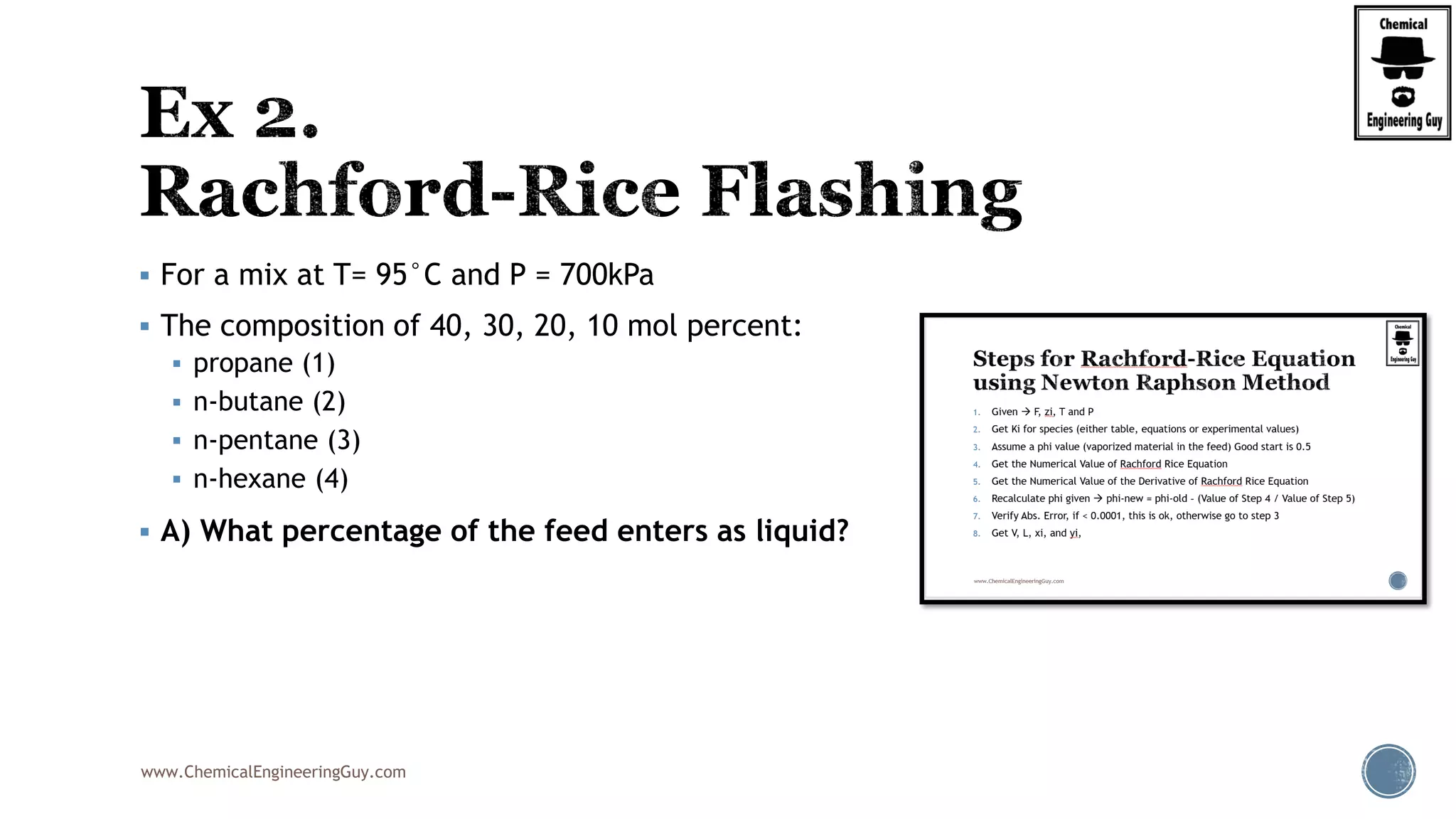
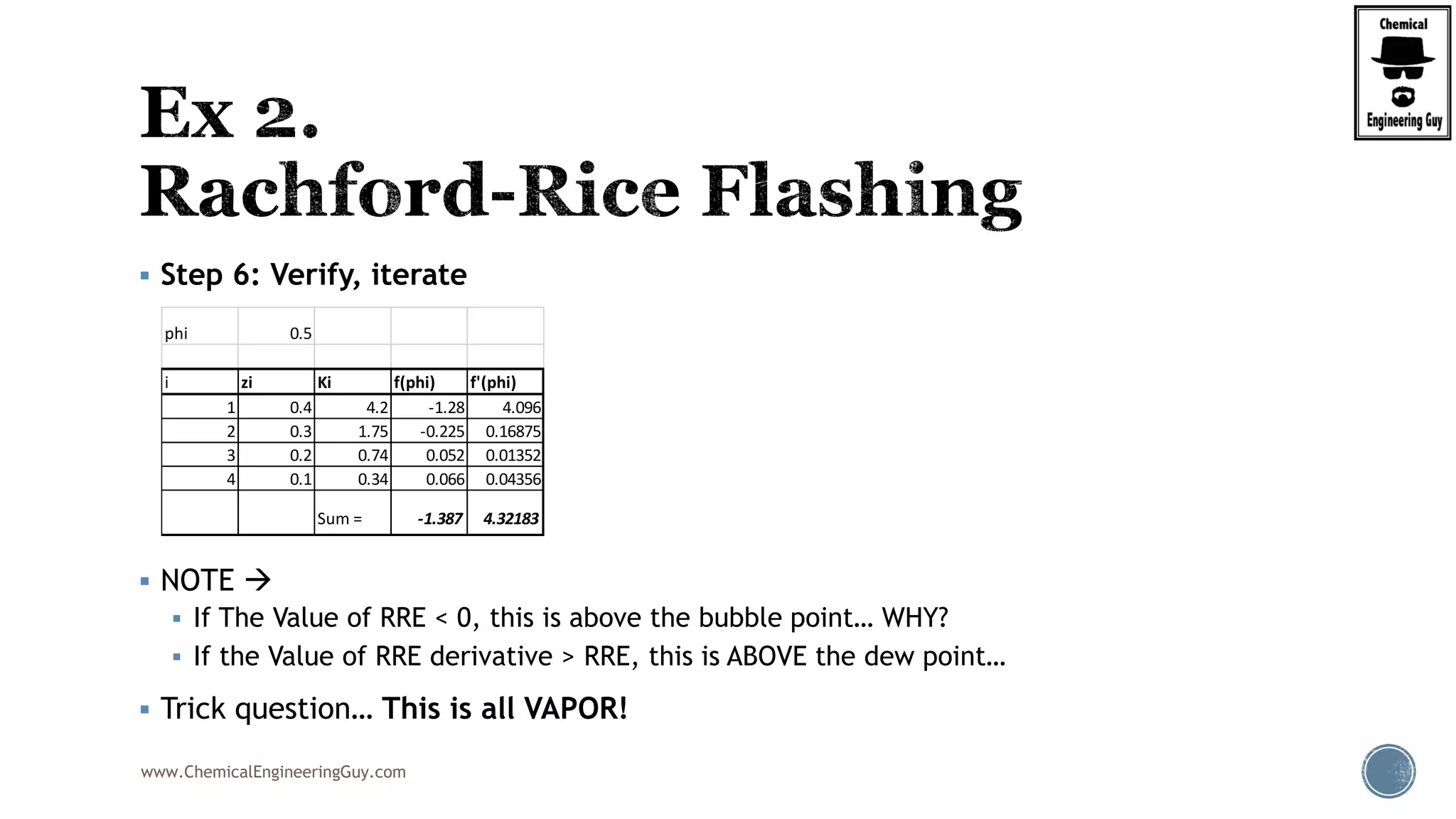
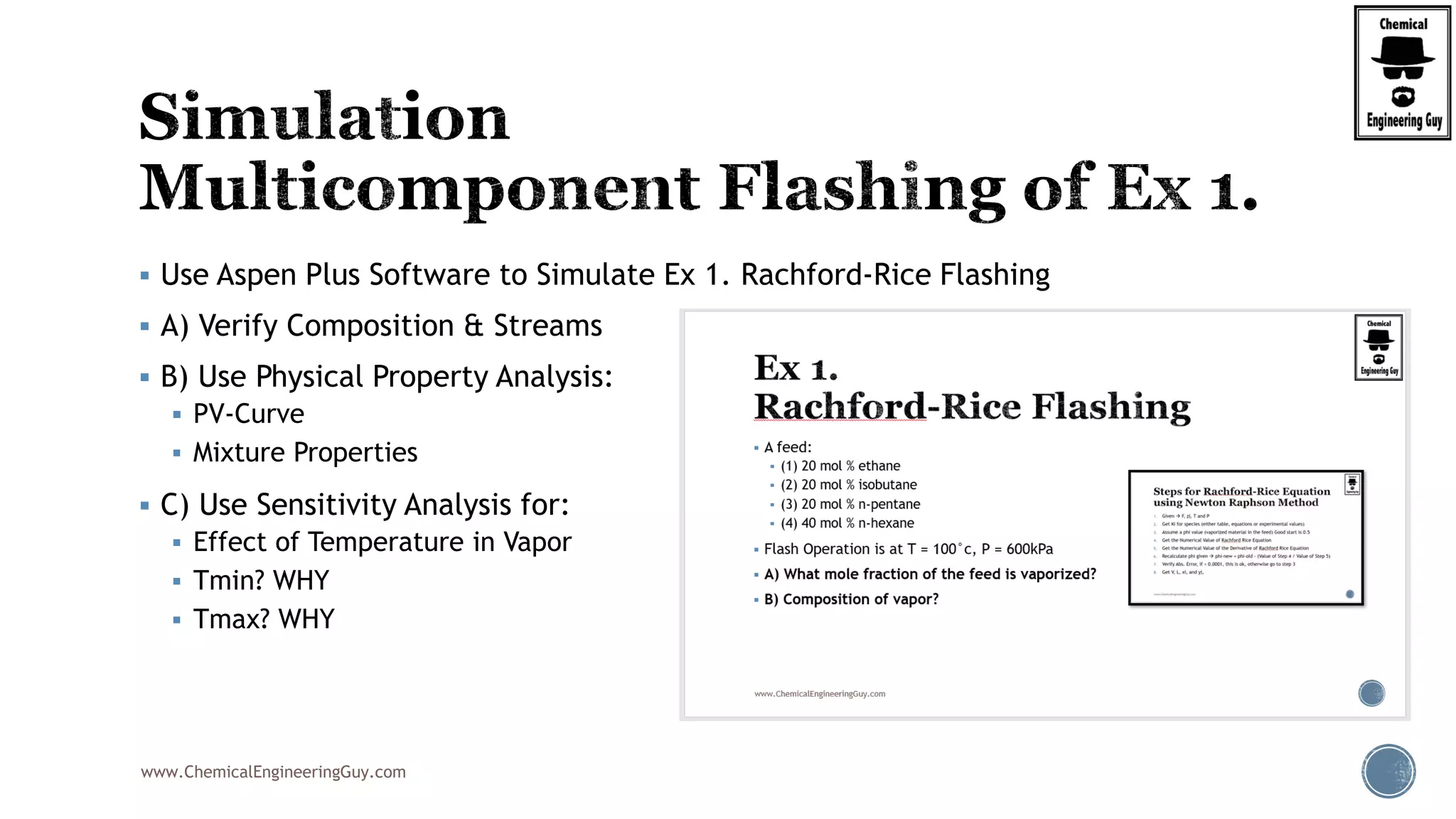
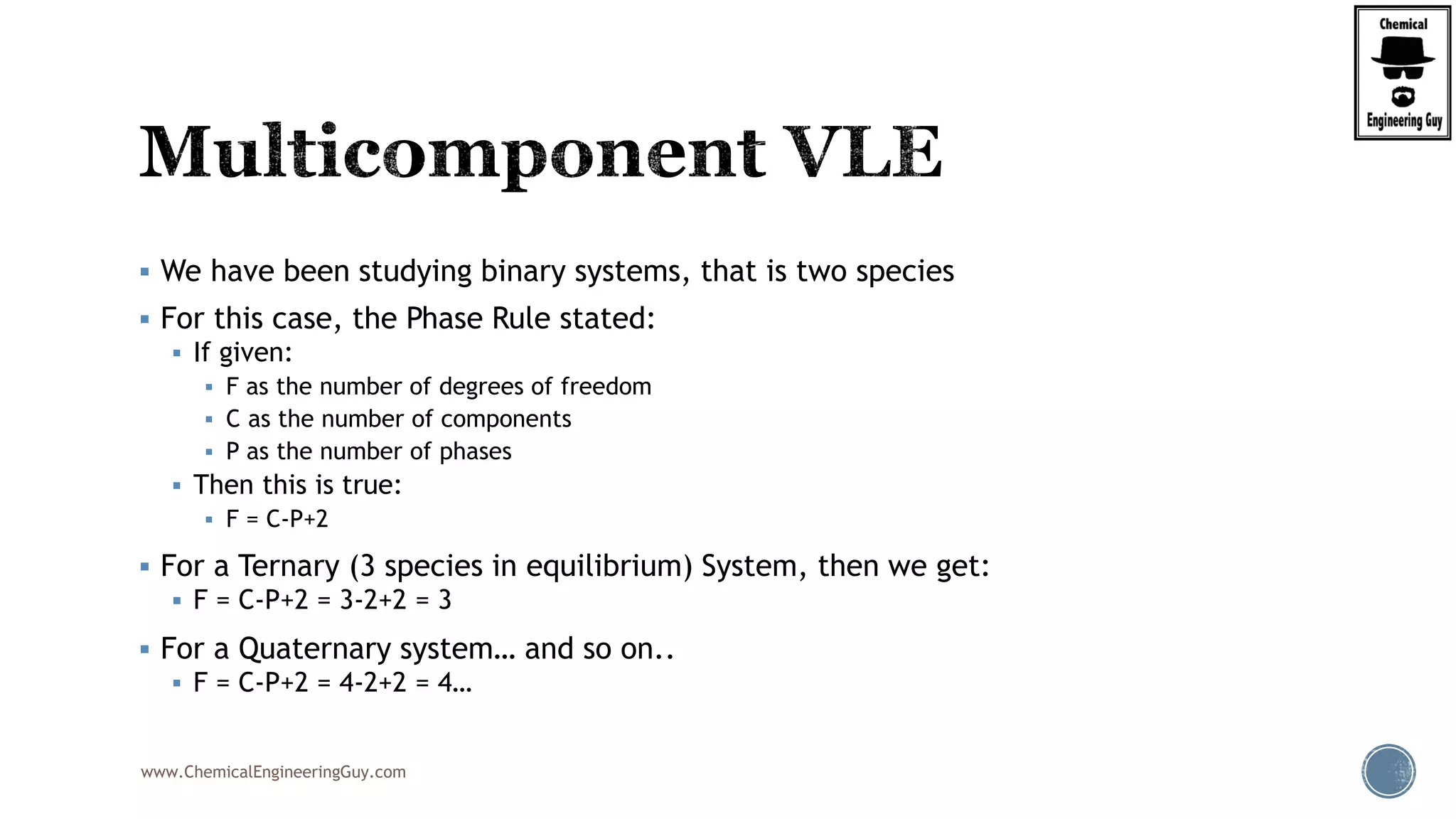
The document discusses the theory and calculations for multicomponent vapor-liquid equilibrium (VLE) and flash distillation, focusing on bubble and dew points and k-values for hydrocarbon systems. It explains the relationship between different phases and the phase rule while emphasizing methods for calculating bubble point and dew point conditions. Additionally, it provides practical examples and methodologies for evaluating hydrocarbon mixtures at specified temperatures and pressures.


















![www.ChemicalEngineeringGuy.com
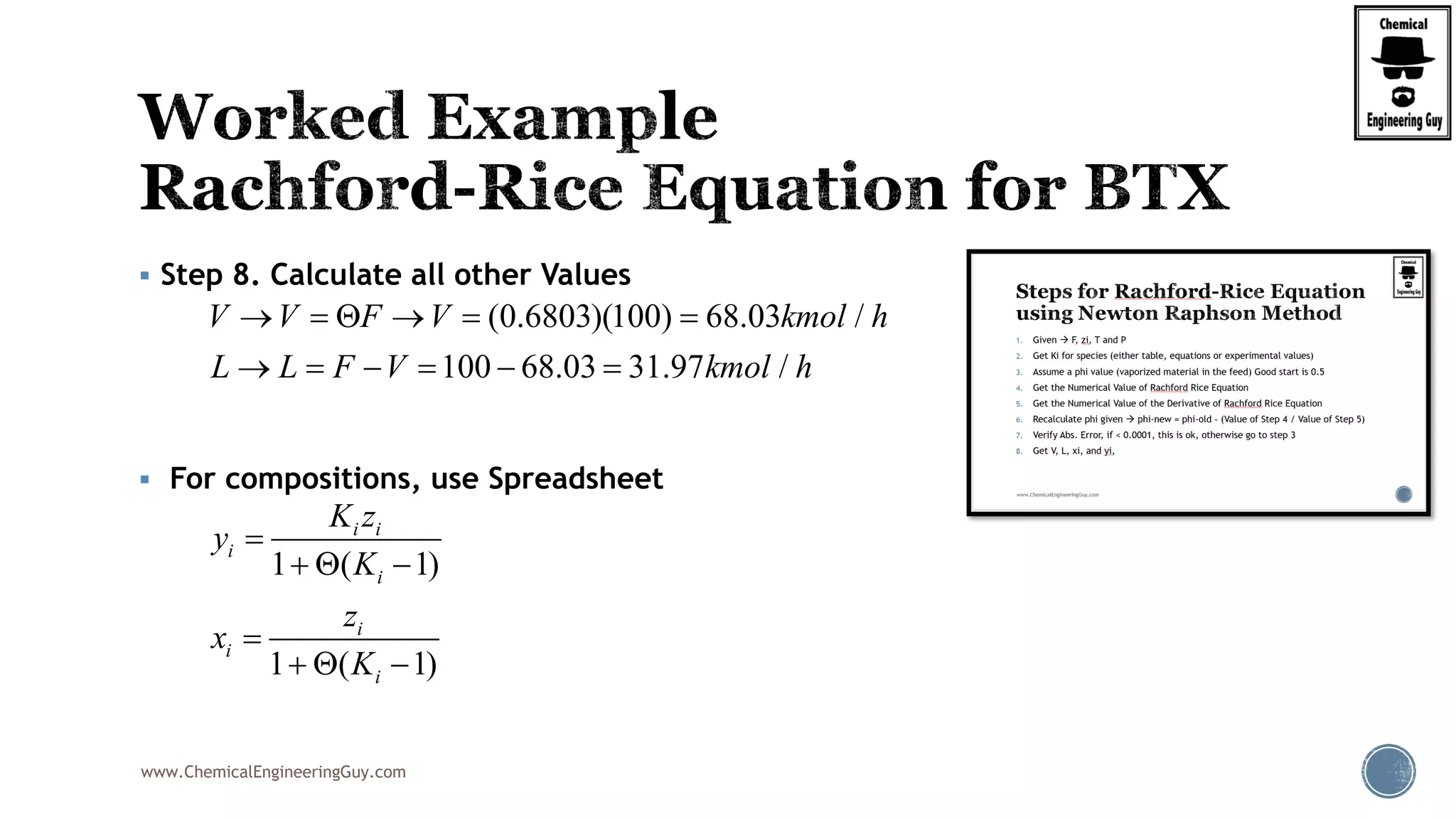
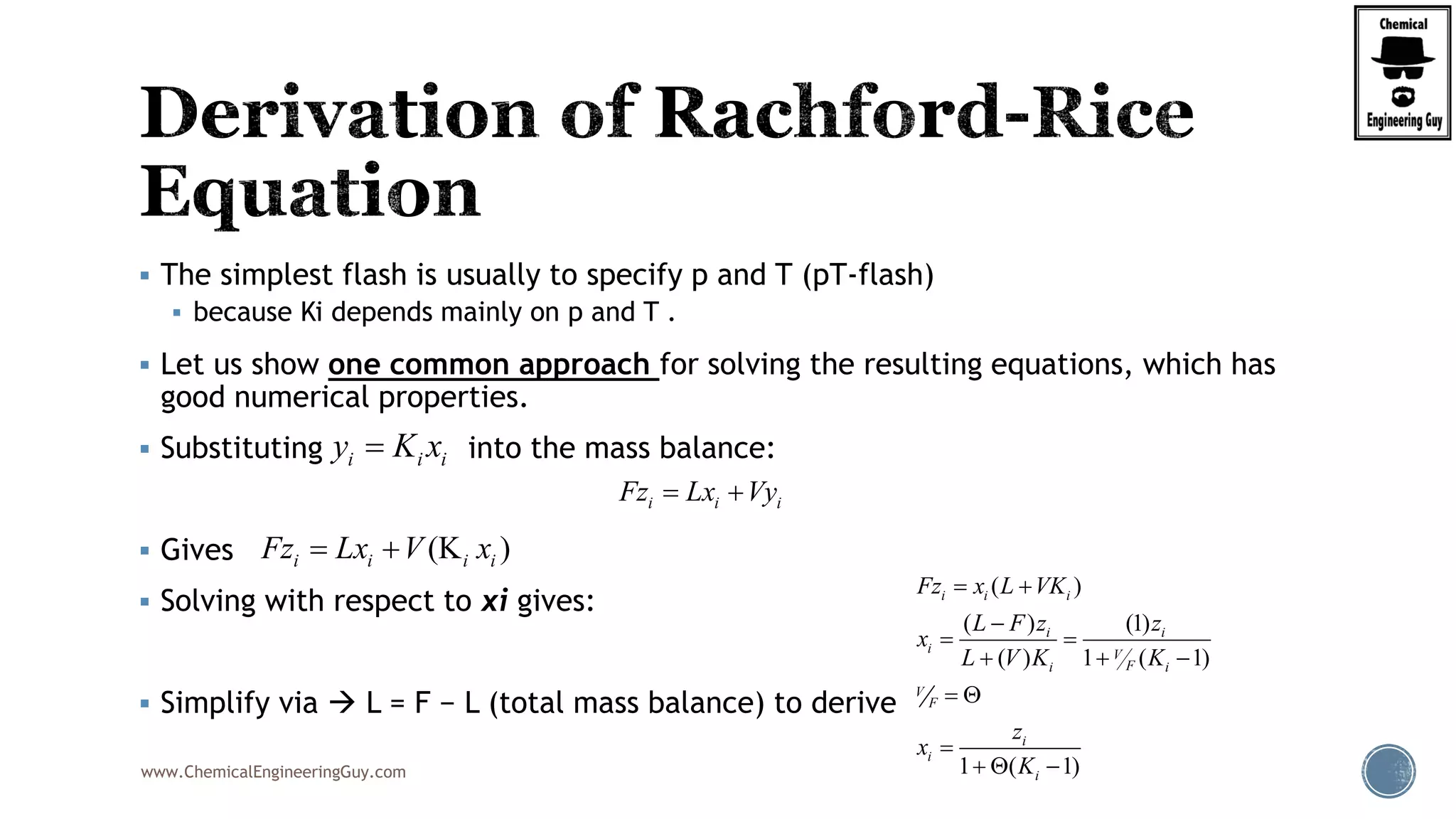
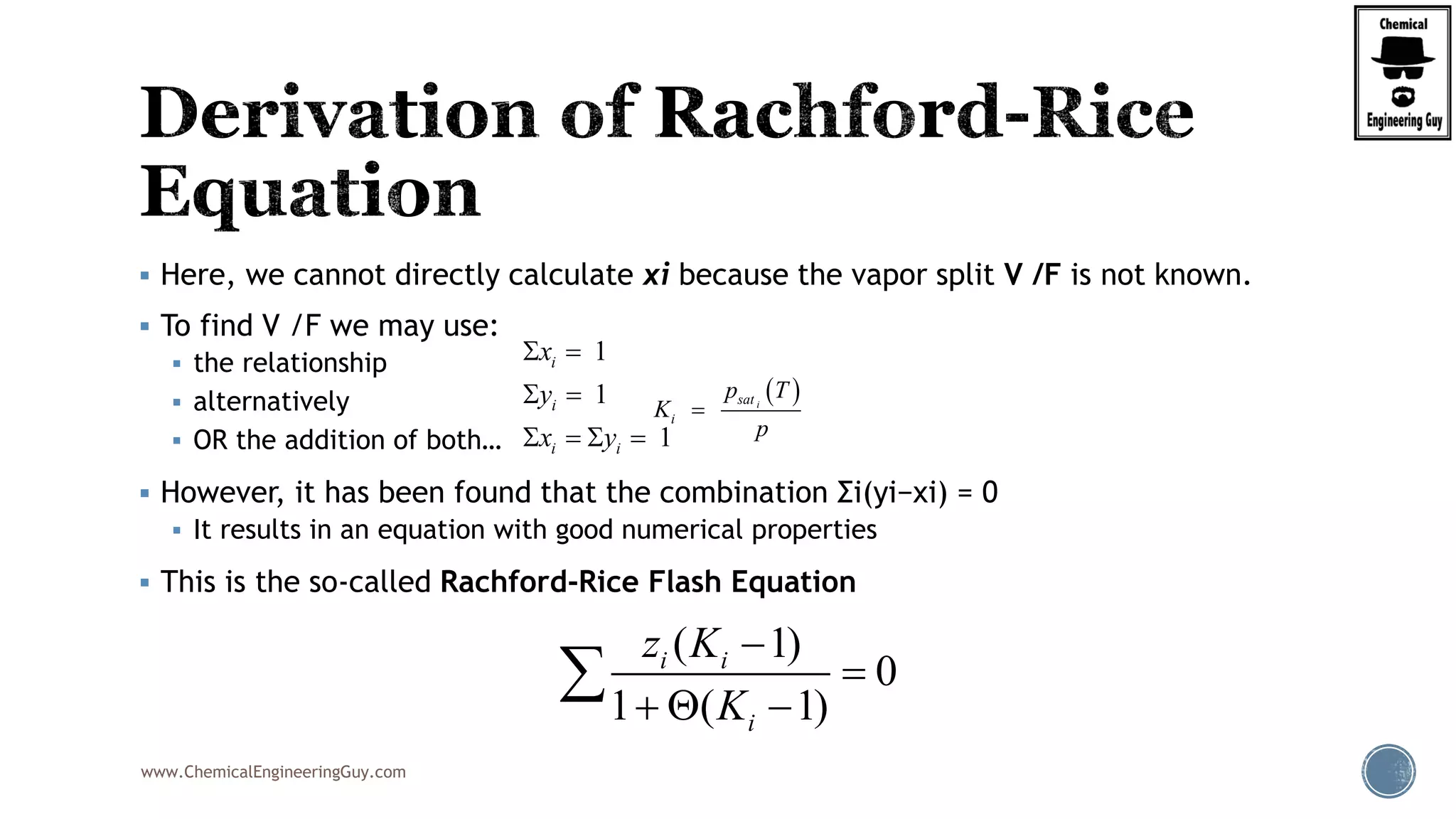
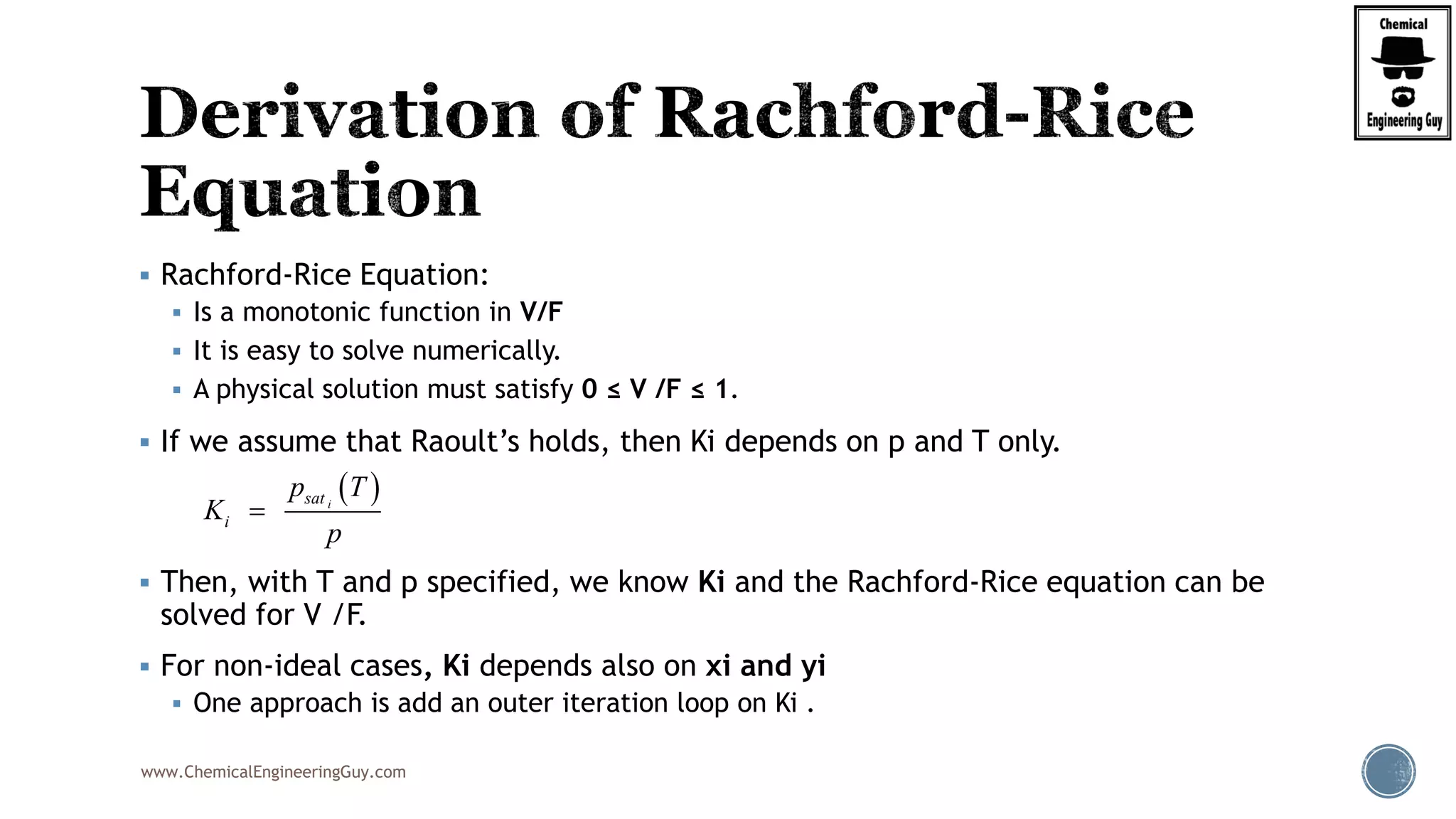
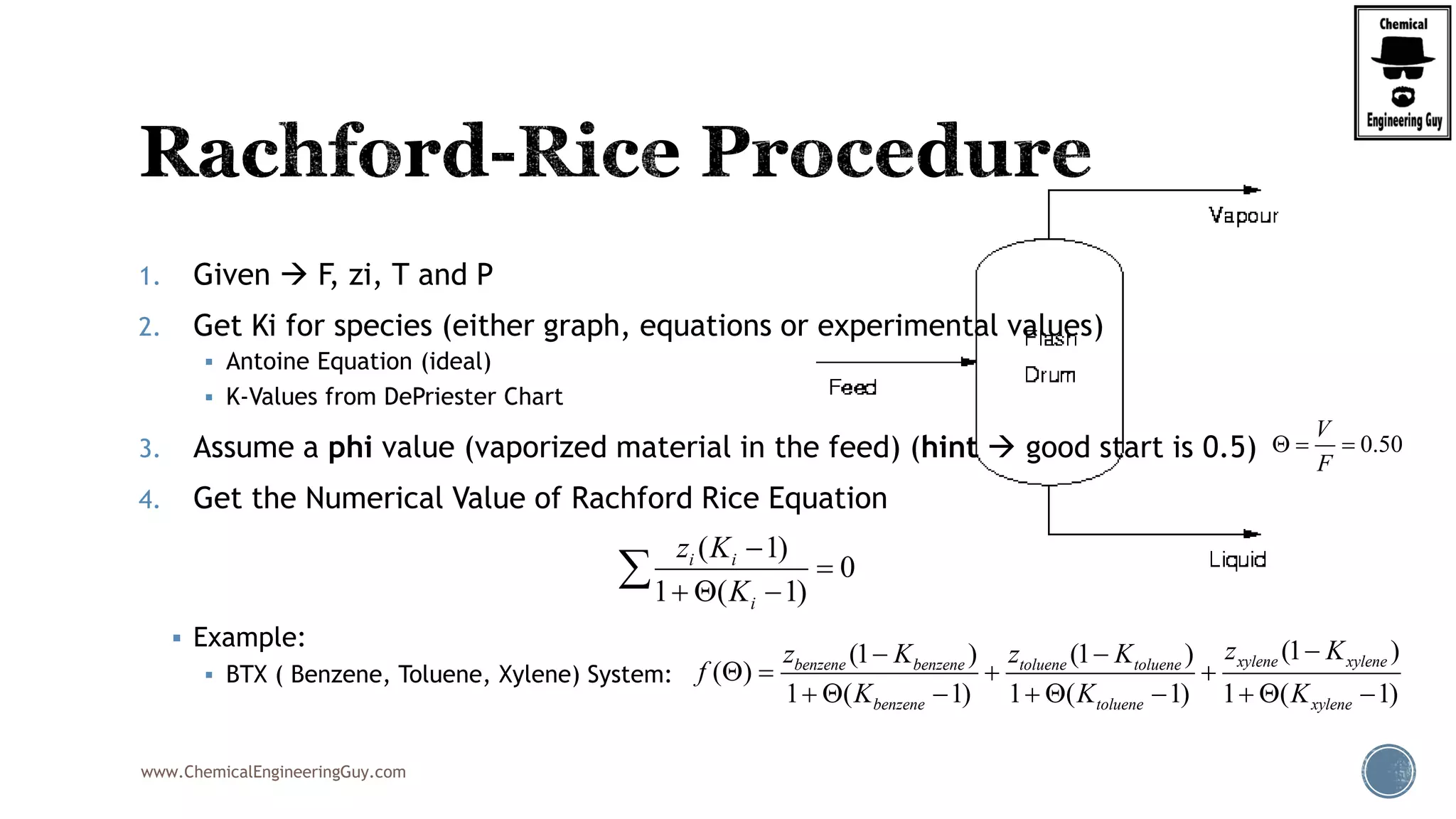
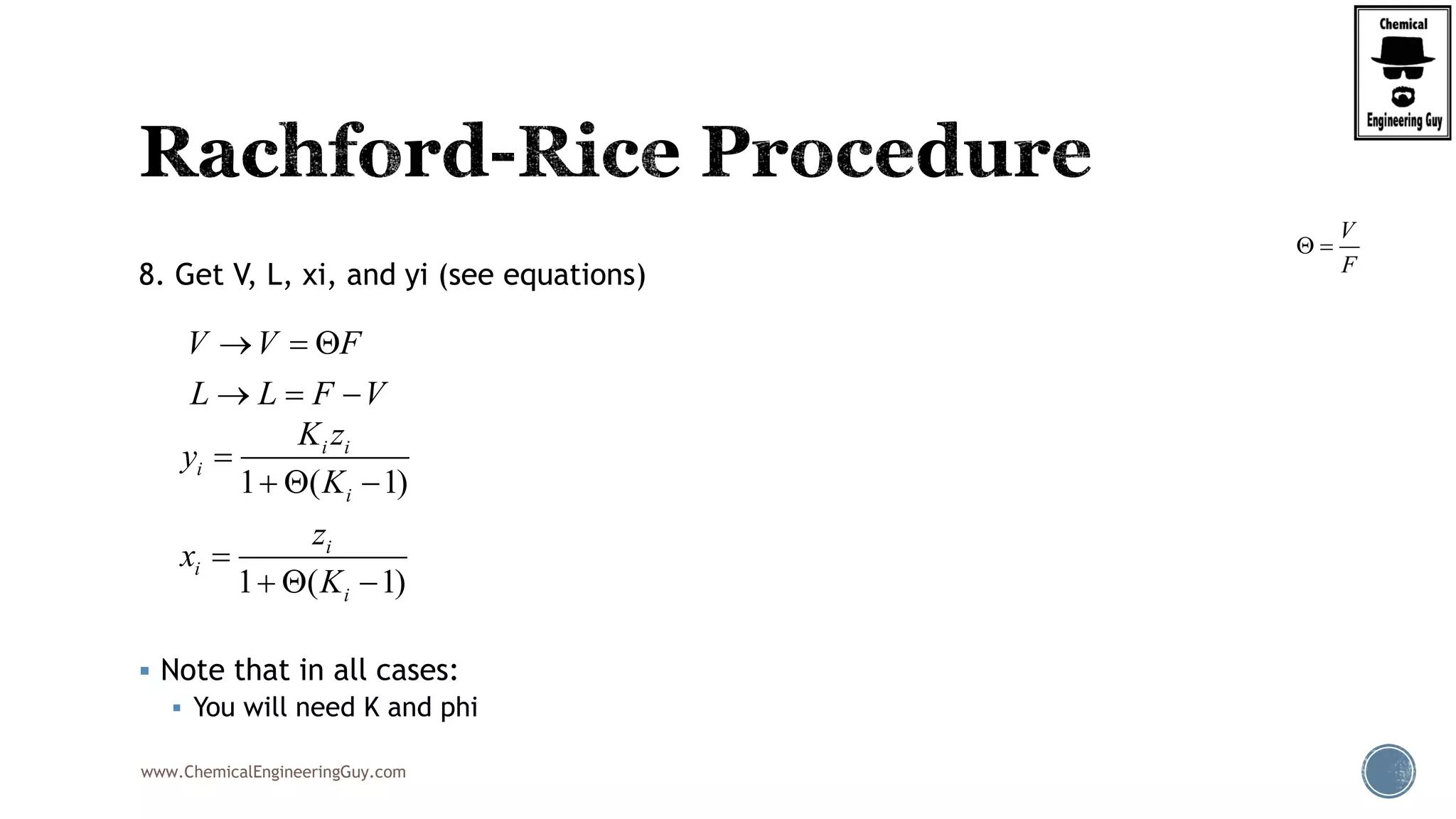
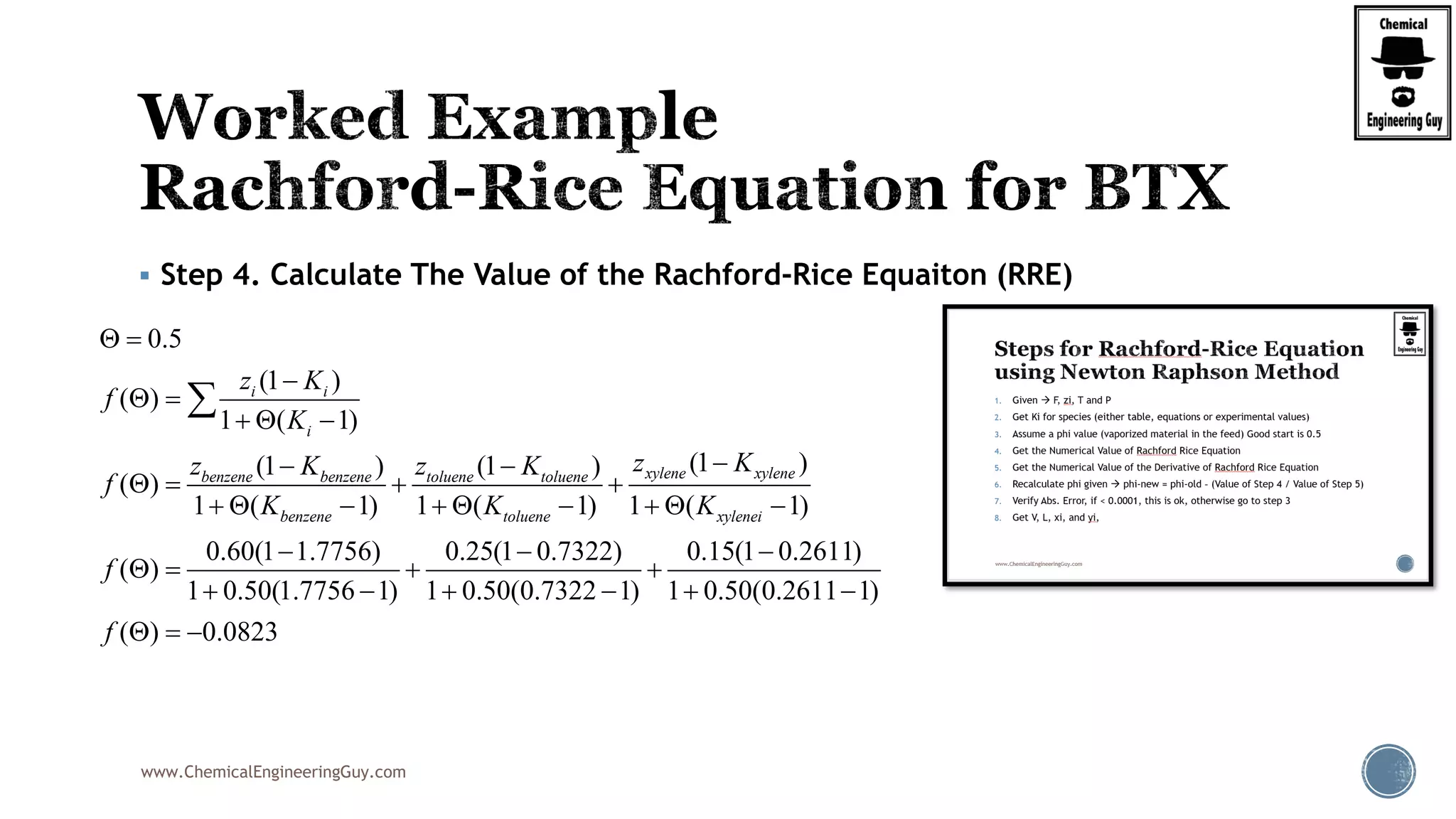
This will be the typical procedure for the RRE
Note that this is based on a numerical method
Newton-Raphson Method
Uses the original function, f(phi)
It also requires the derivative of the function, f’(phi)
( 1)
( )
1 ( 1)
i i
i
z K
f
K
0.50
V
F
2
2
(1 )
'( )
[1 ( 1)]
i i
i
z K
f
K
( , , , ... )i j k zF z z z z
(V,y ,y ,y ...y )i j k z
(L,x ,x ,x ...x )i j k z
( , )T P
( , , ... )i j k zK K K K
Do you need the Full Version?
Contact me if needed!
Contact@ChemicalEngineeringGuy.com
https://courses.chemicalengineeringguy.com/courses
You can also check out more content here:
My Youtube Channel
My Fan Page
The LinkedIn
My website:](https://image.slidesharecdn.com/flashdistillationslideshare3of3-191031011848/75/Flash-Distillation-in-Chemical-and-Process-Engineering-Part-3-of-3-49-2048.jpg)
![www.ChemicalEngineeringGuy.com
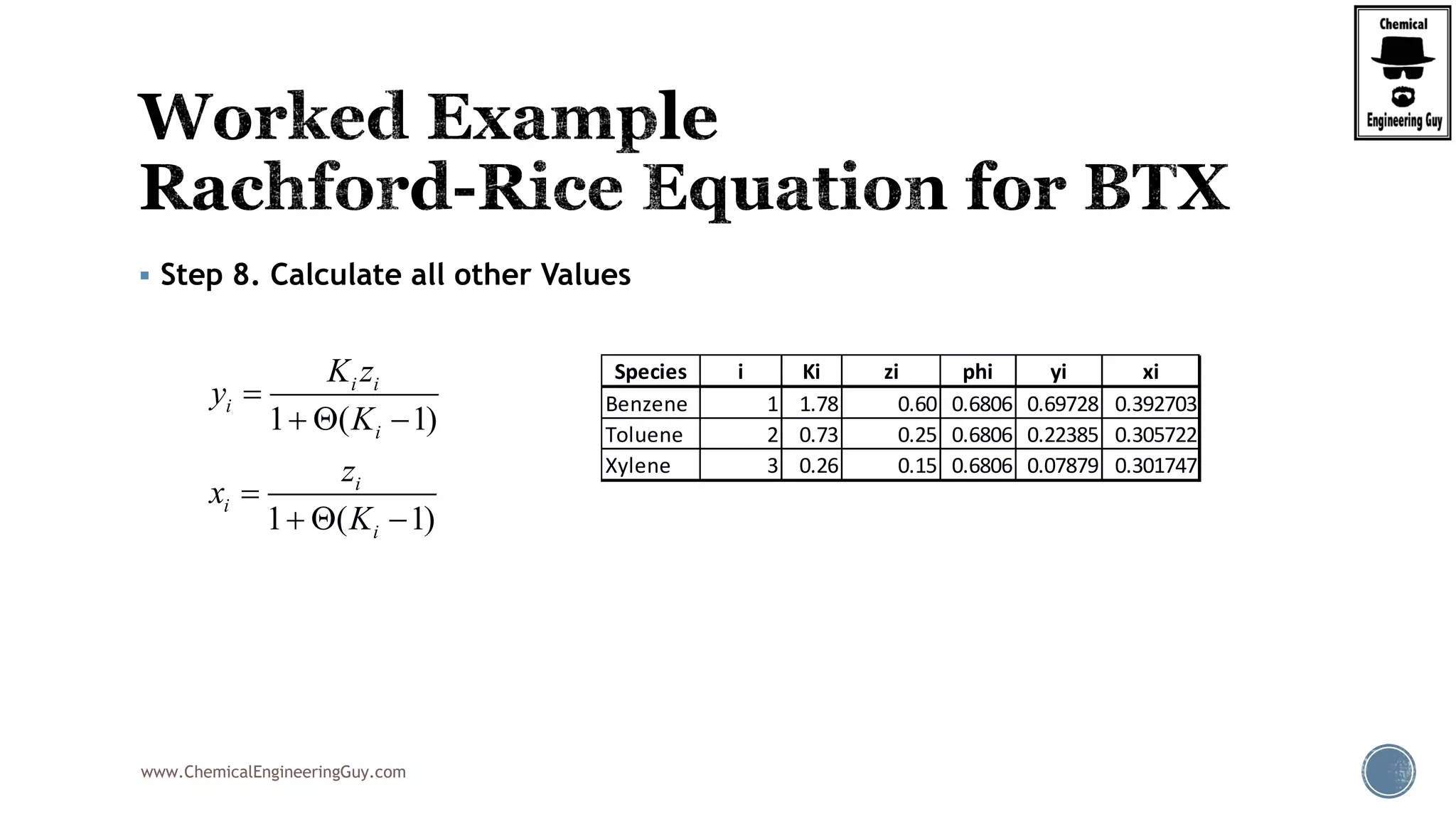
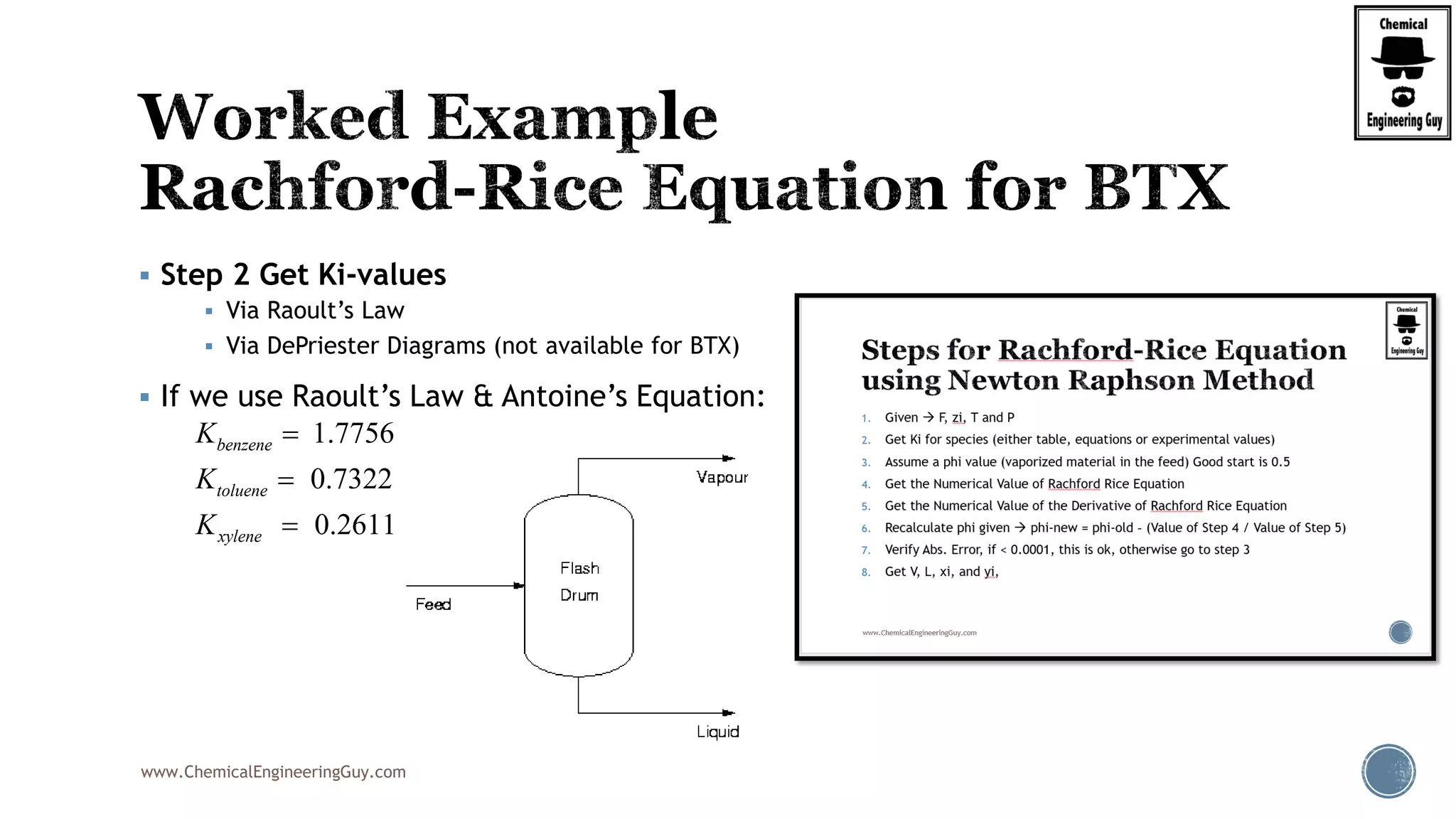
5. Get the Numerical Value of the Derivative of Rachford Rice Equation
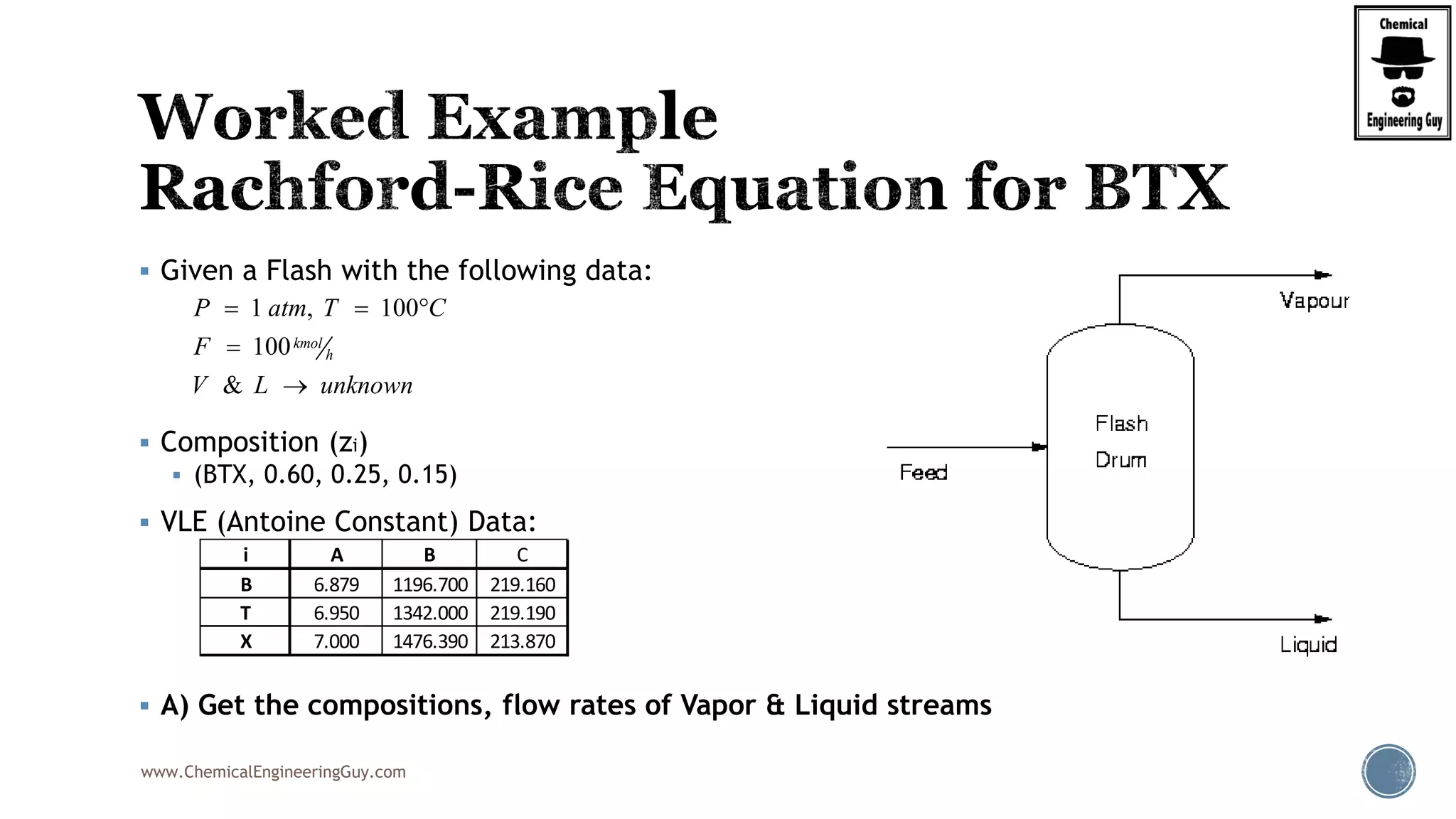
Example:
BTX ( Benzene, Toluene, Xylene) System:
V
F
22 2
2 2 2
(1 )(1 ) (1 )
'( )
[1 ( 1)] [1 ( 1)] [1 ( 1)]
xylene xylenebenzene benzene toluene toluene
benzene toluene xylenei
z Kz K z K
f
K K K
2
2
(1 )
'( )
[1 ( 1)]
i i
i
z K
f
K
](https://image.slidesharecdn.com/flashdistillationslideshare3of3-191031011848/75/Flash-Distillation-in-Chemical-and-Process-Engineering-Part-3-of-3-51-2048.jpg)


![www.ChemicalEngineeringGuy.com
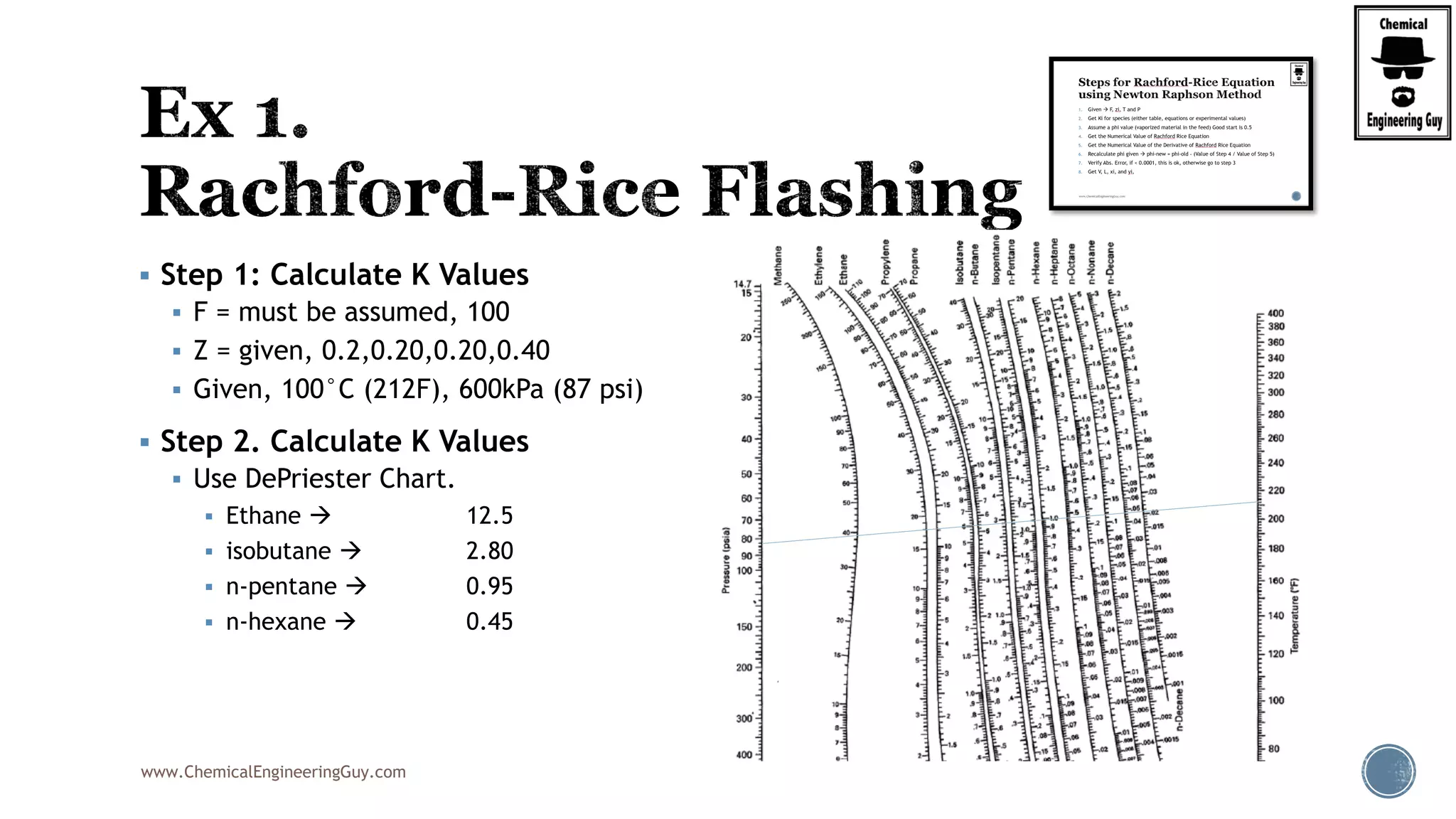
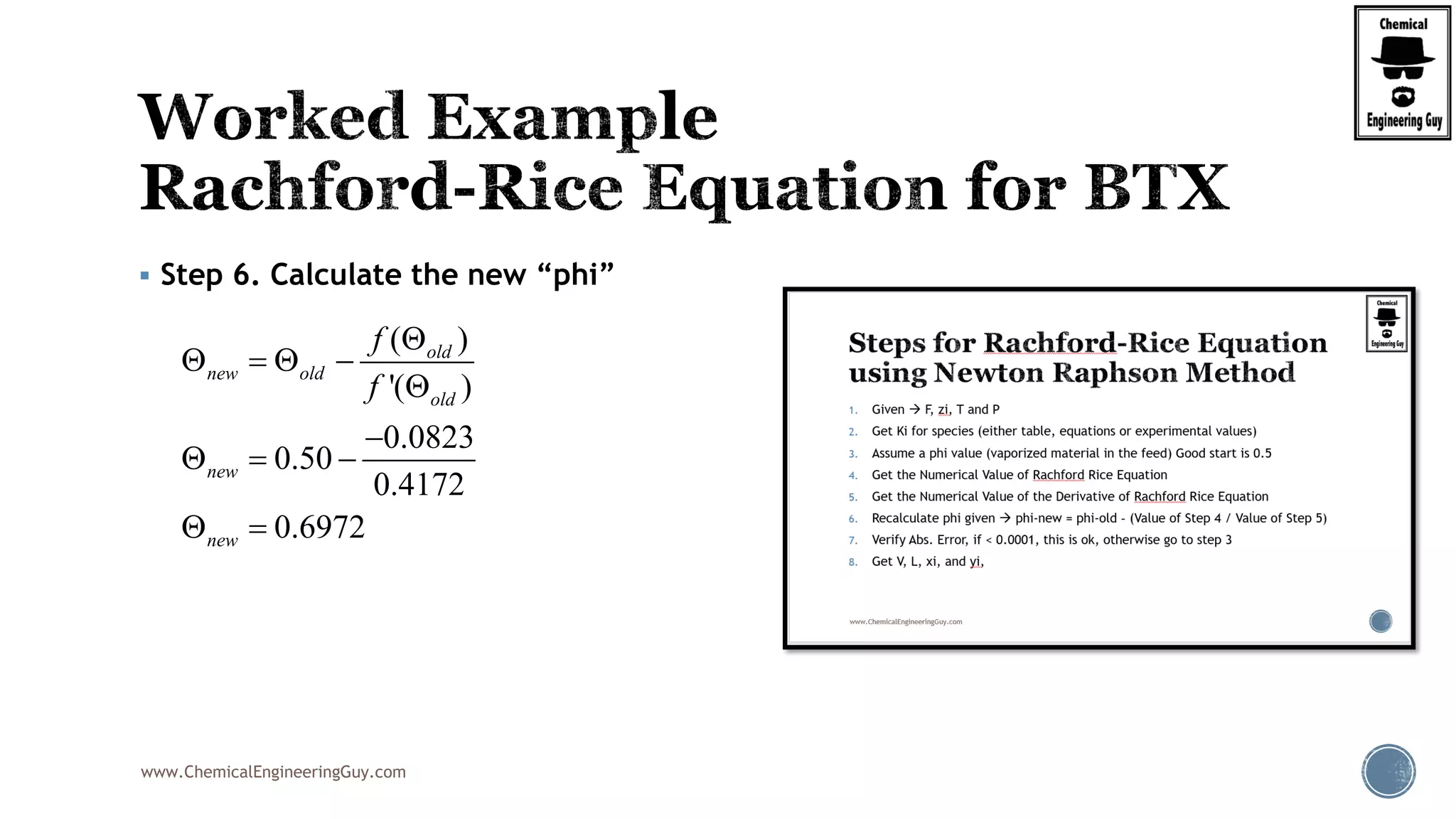
Step 5. Calculate The Value of the derivative of RRE
2
2
22 2
2 2 2
2
2
0.5
(1 )
'( )
[1 ( 1)]
(1 )(1 ) (1 )
'( )
[1 ( 1)] [1 ( 1)] [1 ( 1)]
0.60(1 1.7756) 0.25
'( )
1 0.50(1.7756 1)]
i i
i
xylene xylenebenzene benzene toluene toluene
benzene toluene xylenei
z K
f
K
z Kz K z K
f
K K K
f
2 2
2 2
(1 0.7322) 0.15(1 0.2611)
1 0.50(0.7322 1)] 1 0.50(0.2611 1)]
'( ) 0.4172f
Do you need the Full Version?
Contact me if needed!
Contact@ChemicalEngineeringGuy.com
https://courses.chemicalengineeringguy.com/courses
You can also check out more content here:
My Youtube Channel
My Fan Page
The LinkedIn
My website:](https://image.slidesharecdn.com/flashdistillationslideshare3of3-191031011848/75/Flash-Distillation-in-Chemical-and-Process-Engineering-Part-3-of-3-59-2048.jpg)
![www.ChemicalEngineeringGuy.com
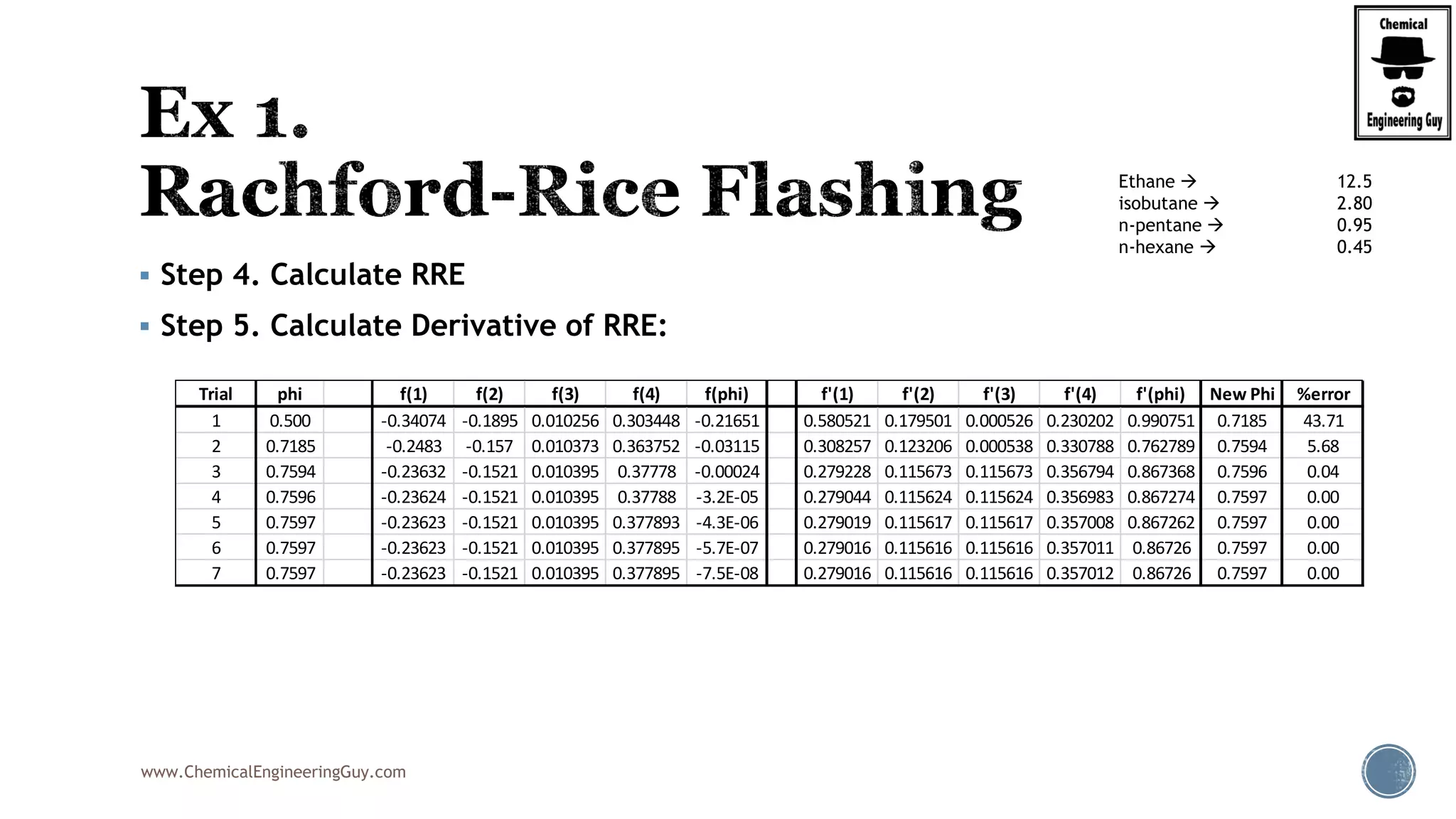
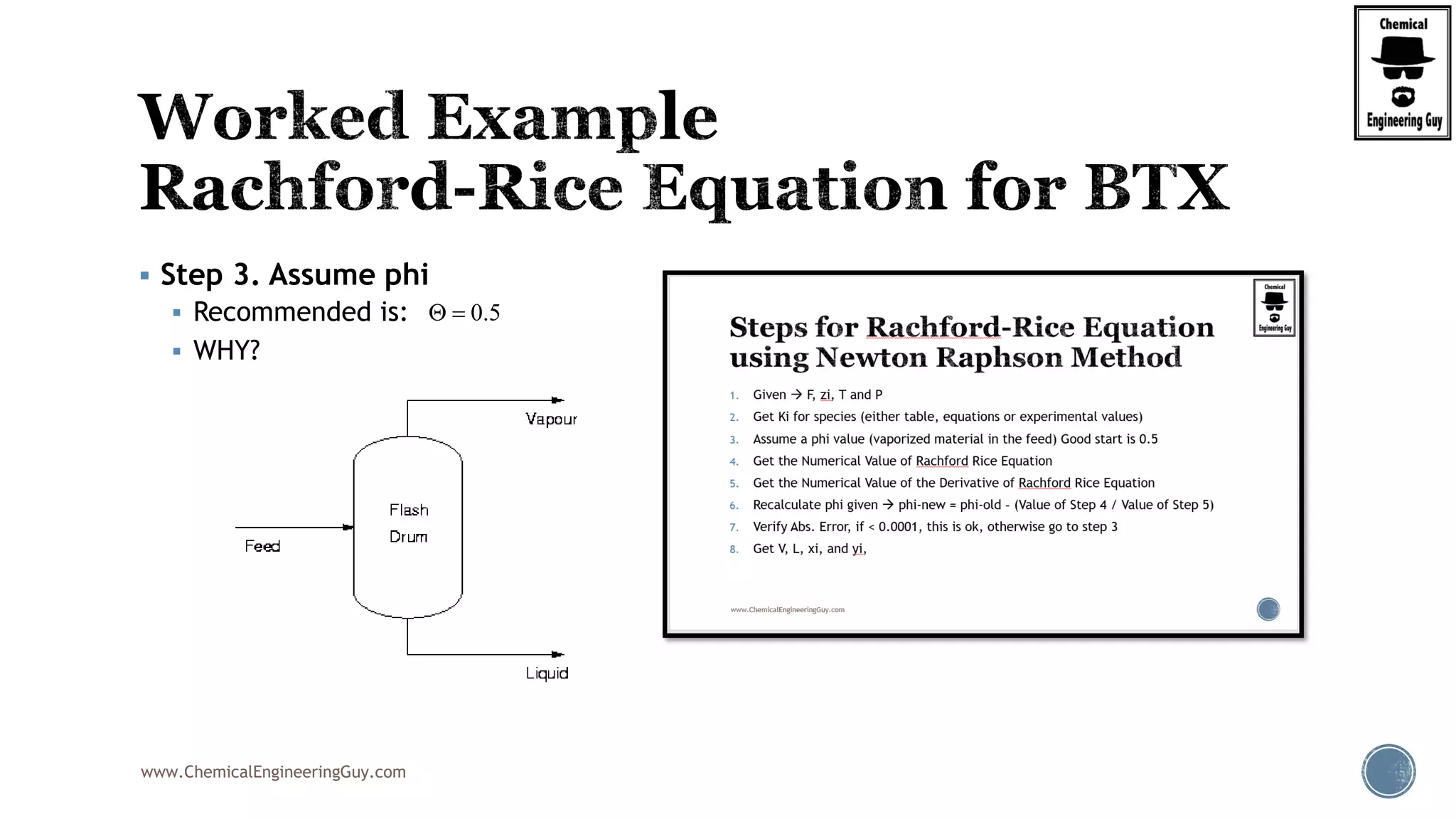
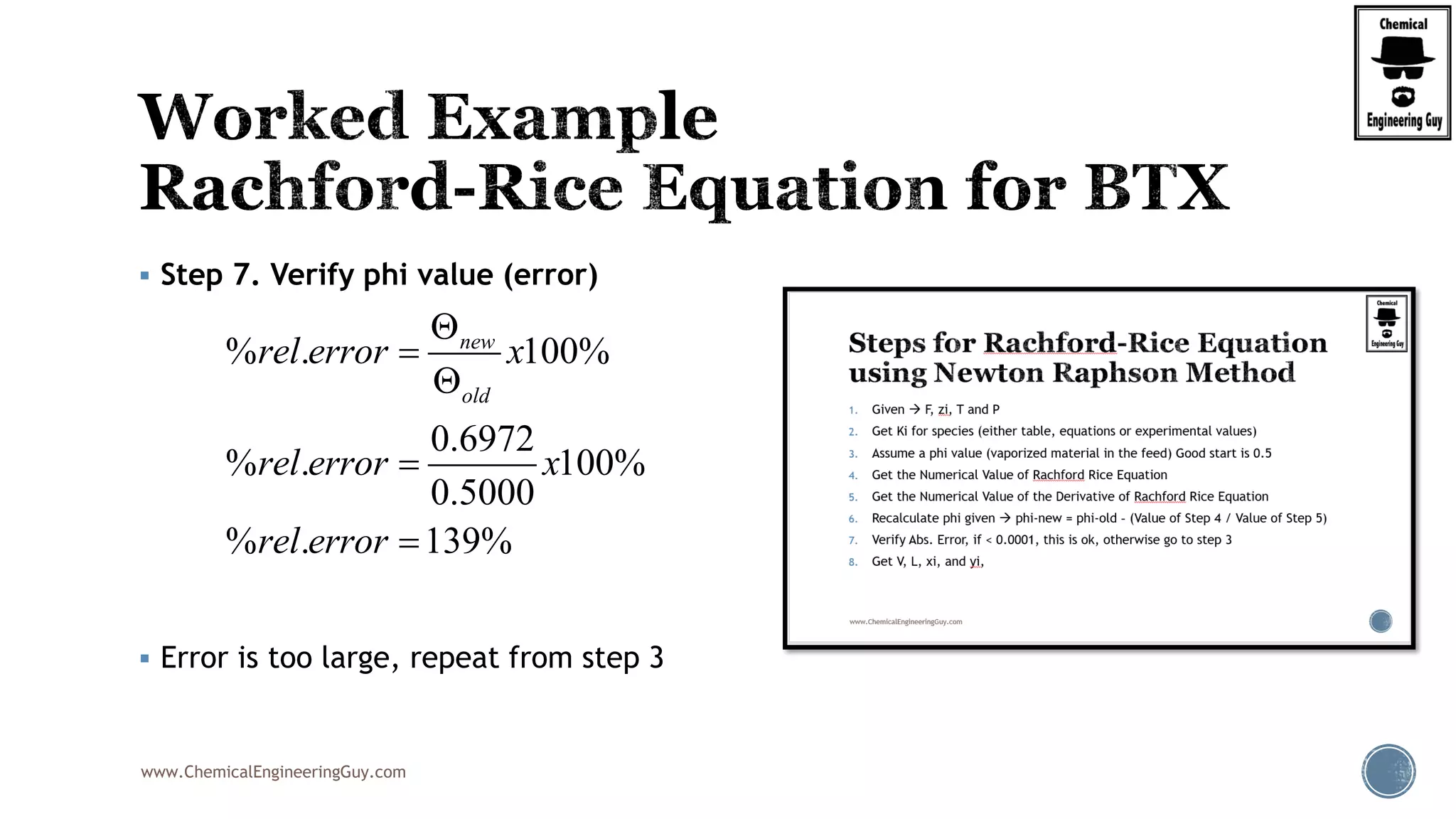
The most convenient way to do this is via a Spreadsheet… as we will need to iterate
Step 7. Verify phi value (error)
Best Case phi = 0.6806; error is acceptable
Trial phi f(1) f(2) f(3) f(phi) f'(1) f'(2) f'(3) f'(phi) New Phi %error
1 0.5 -0.33532 0.0773 0.175775 -0.08225 0.187402 0.023901 0.205979 0.417282 0.6971 39.42
2 0.6971 -0.30205 0.08232 0.228567 0.008834 0.152057 0.027105 0.348286 0.527447 0.6804 2.40
3 0.6804 -0.30462 0.08187 0.222879 0.000126 0.154654 0.026808 0.026808 0.20827 0.6797 0.09
4 0.6797 -0.30471 0.08185 0.222679 -0.00018 0.154749 0.026797 0.026797 0.208344 0.6806 0.13
5 0.6806 -0.30458 0.08187 0.222971 0.000269 0.154611 0.026813 0.026813 0.208236 0.6793 0.19
6 0.6793 -0.30478 0.08184 0.222544 -0.00039 0.154813 0.02679 0.02679 0.208394 0.6812 0.28
7 0.6812 -0.30448 0.08189 0.223167 0.000572 0.154518 0.026823 0.026823 0.208164 0.6785 0.40
2
2
22 2
2 2 2
2
2
0.5
(1 )
'( )
[1 ( 1)]
(1 )(1 ) (1 )
'( )
[1 ( 1)] [1 ( 1)] [1 ( 1)]
0.60(1 1.7756) 0.25
'( )
1 0.50(1.7756 1)]
i i
i
xylene xylenebenzene benzene toluene toluene
benzene toluene xylenei
z K
f
K
z Kz K z K
f
K K K
f
2 2
2 2
(1 0.7322) 0.15(1 0.2611)
1 0.50(0.7322 1)] 1 0.50(0.2611 1)]
'( ) 0.4172f
f’(1) f’(2) f’(3)
0.5
(1 )
( )
1 ( 1)
(1 )(1 ) (1 )
( )
1 ( 1) 1 ( 1) 1 ( 1)
0.60(1 1.7756) 0.25(1 0.7322)
( )
1 0.50(1.7756 1) 1 0.50(0.732
i i
i
xylene xylenebenzene benzene toluene toluene
benzene toluene xylenei
z K
f
K
z Kz K z K
f
K K K
f
0.15(1 0.2611)
2 1) 1 0.50(0.2611 1)
( ) 0.0823f
f(1) f(2) f(3)
Do you need the Full Version?
Contact me if needed!
Contact@ChemicalEngineeringGuy.com
https://courses.chemicalengineeringguy.com/courses
You can also check out more content here:
My Youtube Channel
My Fan Page
The LinkedIn
My website:](https://image.slidesharecdn.com/flashdistillationslideshare3of3-191031011848/75/Flash-Distillation-in-Chemical-and-Process-Engineering-Part-3-of-3-62-2048.jpg)