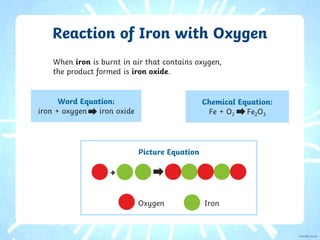
1. The document describes how iron and magnesium react with oxygen through combustion reactions. When iron reacts with oxygen, iron oxide is formed, appearing as a grayish solid. A practical investigation demonstrates how steel wool burns with a yellow flame and leaves behind iron oxide.
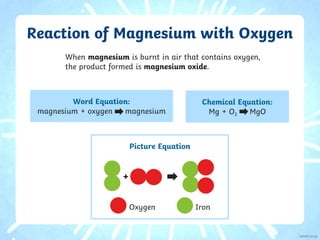
2. Similarly, when magnesium reacts with oxygen, magnesium oxide is produced as a white powder. Magnesium burns with an extremely bright white flame.
3. Both reactions involve the metal combining with oxygen to form an oxide. The balanced chemical equations are provided.