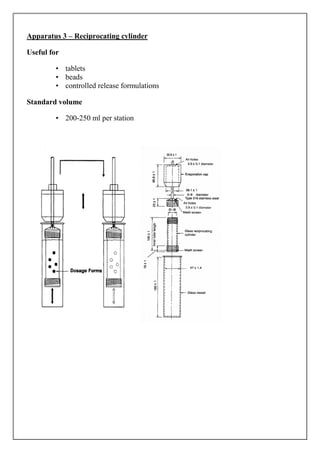
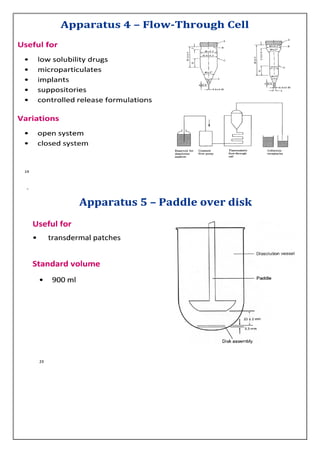
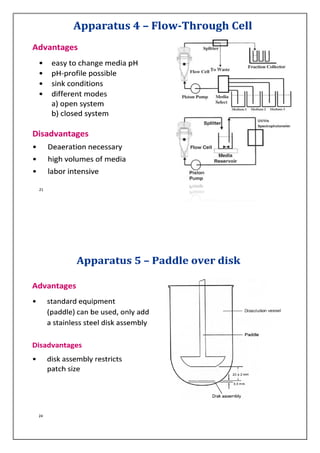
The document discusses dissolution testing of pharmaceutical dosage forms. It defines dissolution and explains why it is an important quality control test. It summarizes the various apparatus used for dissolution testing and the types of dosage forms they are suited for. It also provides details about test conditions and acceptance criteria for different dosage forms like immediate release, delayed release, extended release and transdermal delivery systems.