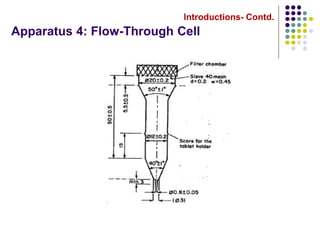
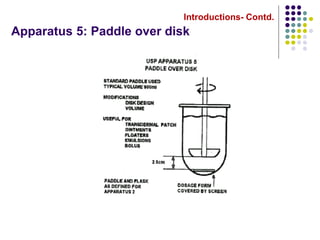
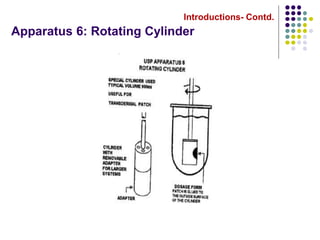
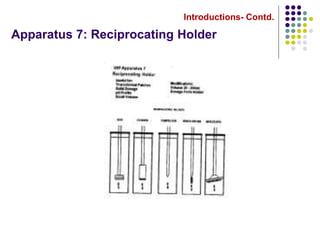
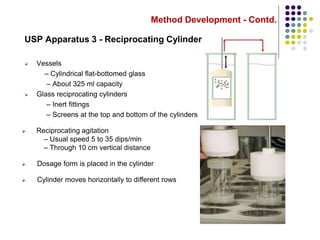
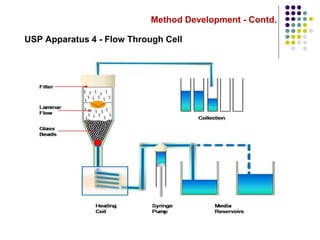
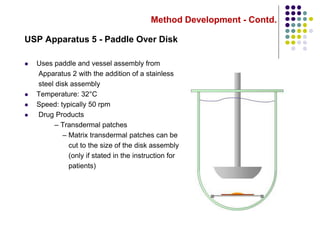
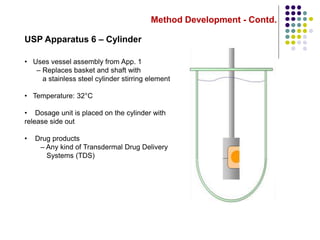
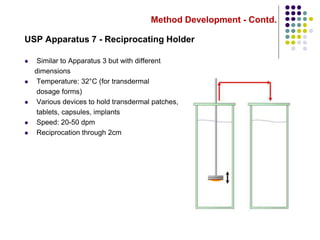
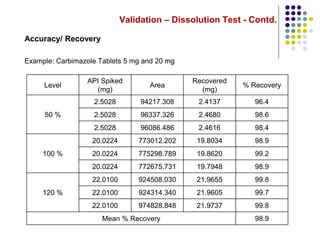
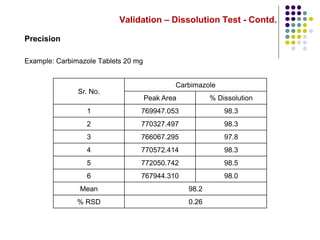
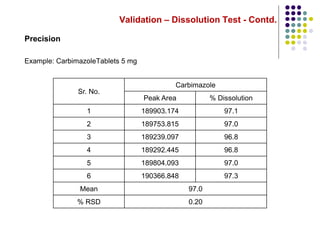
This document discusses dissolution testing techniques used in the pharmaceutical industry. It begins with introductions to dissolution testing, including its history and importance. It then covers development of dissolution methods, including characterizing drug substances and formulations, classifying drugs based on solubility and permeability, and selecting test conditions like apparatus, medium, agitation, and time points. The document discusses compendial and regulatory expectations for dissolution testing as well as validating dissolution methods.