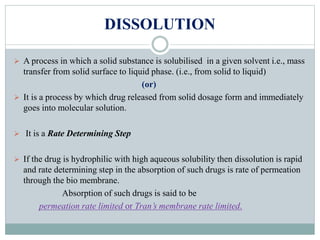
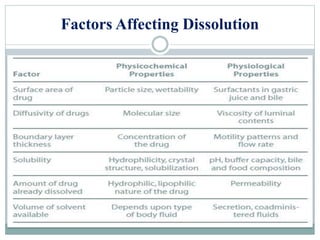
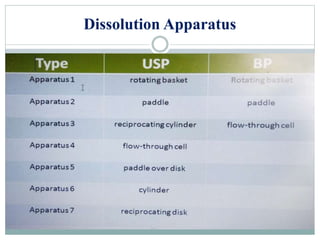
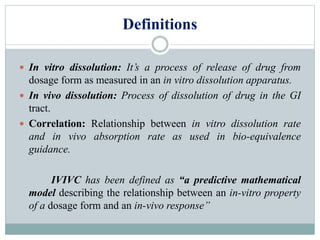
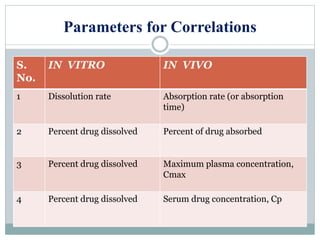
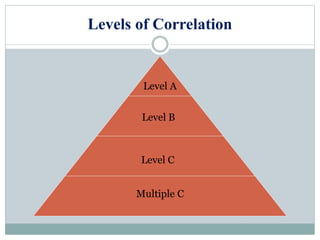
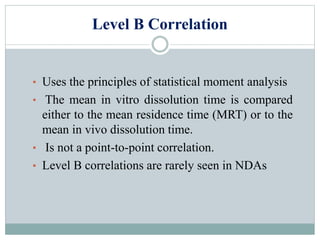
This document discusses in vitro dissolution testing and in vitro-in vivo correlations (IVIVCs). It defines dissolution as the process by which a drug is released from a solid dosage form and solubilized in a liquid solvent. Dissolution is an important quality control procedure linked to in vivo product performance. The document describes different levels of IVIVC (A, B, C, multiple level C) and their regulatory significance. It also discusses using computer models to establish IVIVCs through convolution and deconvolution approaches to predict plasma concentration profiles and estimate in vitro dissolution profiles.