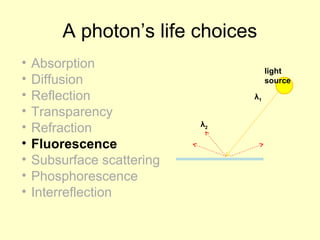
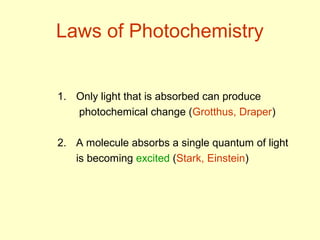
This document discusses photochemistry, which involves chemical reactions that are accompanied by light. It covers the electromagnetic spectrum and defines a photon as a particle of light. Examples of photochemical reactions discussed include chemiluminescence, bioluminescence, and the formation of ozone in the atmosphere through absorption of ultraviolet light. The document also addresses the mechanisms of light absorption by molecules, photodissociation reactions, photosensitized reactions like photosynthesis, and the process of photography.
























![Photosynthesis
The photosynthesis of hydrogen chloride
Overall reaction:
Cl2 + H2 2HCl [no reaction in darkness]](https://image.slidesharecdn.com/photochemistrys-190103061017/85/Photochemistry-s-y-27-320.jpg)




![Ago
AgBr*
developer
reduction
Unactivated granuli will be unaffected (but
photosensitive!)
d) Fixation: Unaffected (photosensitive) AgBr should
be removed:
AgBr + 2S2O3
2- [Ag(S2O3)2]3- + Br -
c) Development: Treating the exposed film with a
mild reducing agent the activated granuli will
accelerate the reduction to metallic silver (black)](https://image.slidesharecdn.com/photochemistrys-190103061017/85/Photochemistry-s-y-32-320.jpg)



