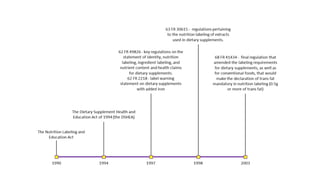
Dietary supplements in the nutraceuticals industry must comply with FDA regulations established by the Dietary Supplement Health and Education Act of 1994, which does not require FDA safety review before marketing. Manufacturers are responsible for ensuring products are not misbranded or adulterated and must avoid making disease treatment claims on labels, which can result in classification as unapproved new drugs. Regulations vary globally, with specific labeling and safety responsibilities in Europe and India, each ensuring proper consumer information and warnings on dietary supplements.