Embed presentation
Downloaded 12 times

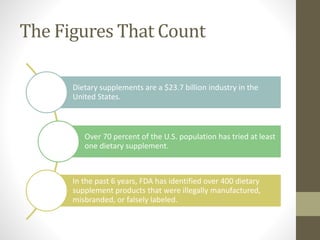


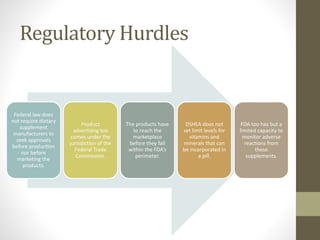
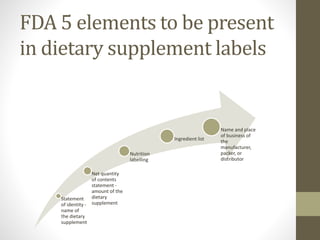

The dietary supplements industry in the U.S. is valued at $23.7 billion, with over 70% of the population having used at least one supplement. The FDA has identified over 400 poorly manufactured or misbranded products in recent years, while federal law does not require pre-market approval for dietary supplements. The Dietary Supplements Health and Education Act (DSHEA) defines dietary supplements and outlines labeling requirements, but regulatory oversight for adverse reactions is limited.






