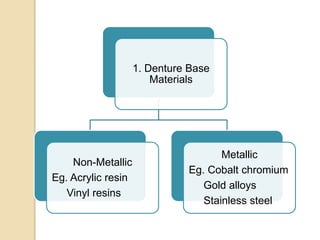
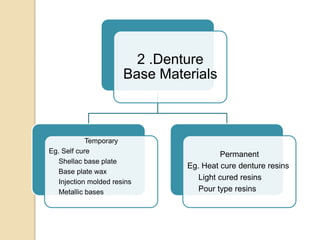
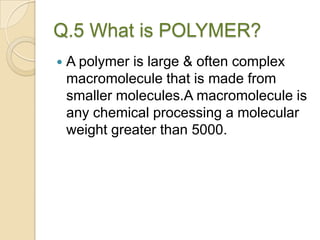
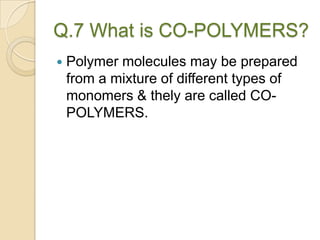
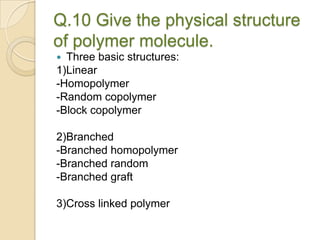
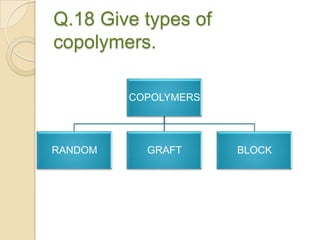
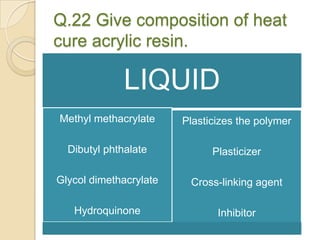
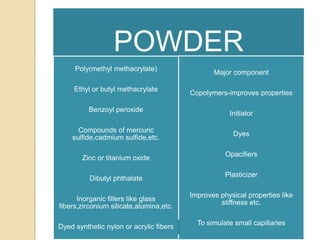
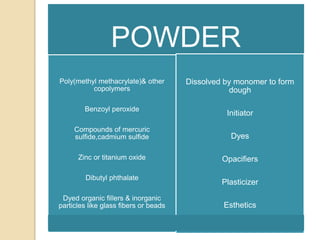
This document discusses denture base materials, specifically acrylic resins. It begins by defining denture base and classifying denture base resins as non-metallic, metallic, temporary or permanent. Ideal requirements of dental resins are listed. Composition and differences between heat cure and self cure acrylic resins are provided. Processing techniques like compression molding and the curing cycle are described. Other resin types like light activated are also mentioned. Common processing errors in acrylic resins like porosity, crazing and warpage are listed.