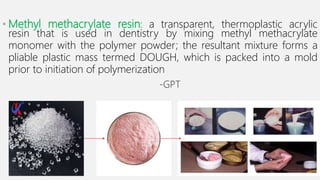
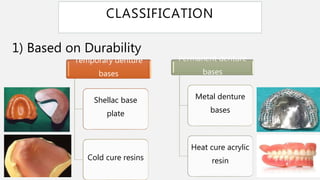
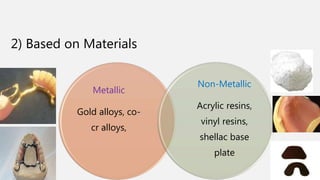
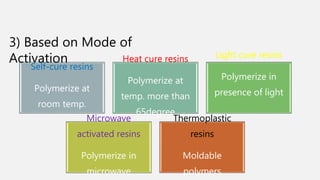
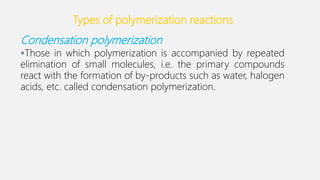
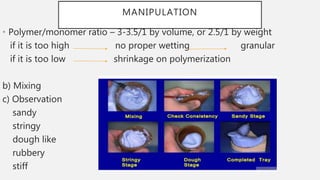
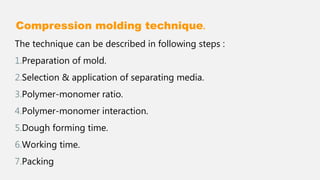
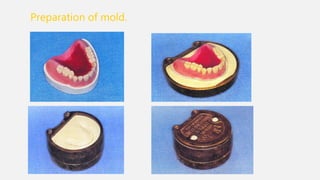
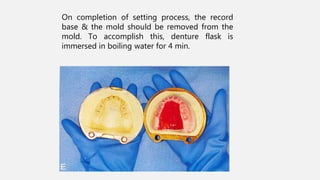
The document discusses denture base resins, including their history, properties, classifications, and advancements in materials used in dentistry. It highlights the evolution from vulcanite to polymethyl methacrylate (PMMA), and details various types of resins, their polymerization processes, and requirements for ideal denture base materials. The conclusion reflects on the promising advancements in denture resins and potential future applications to improve dental treatments.