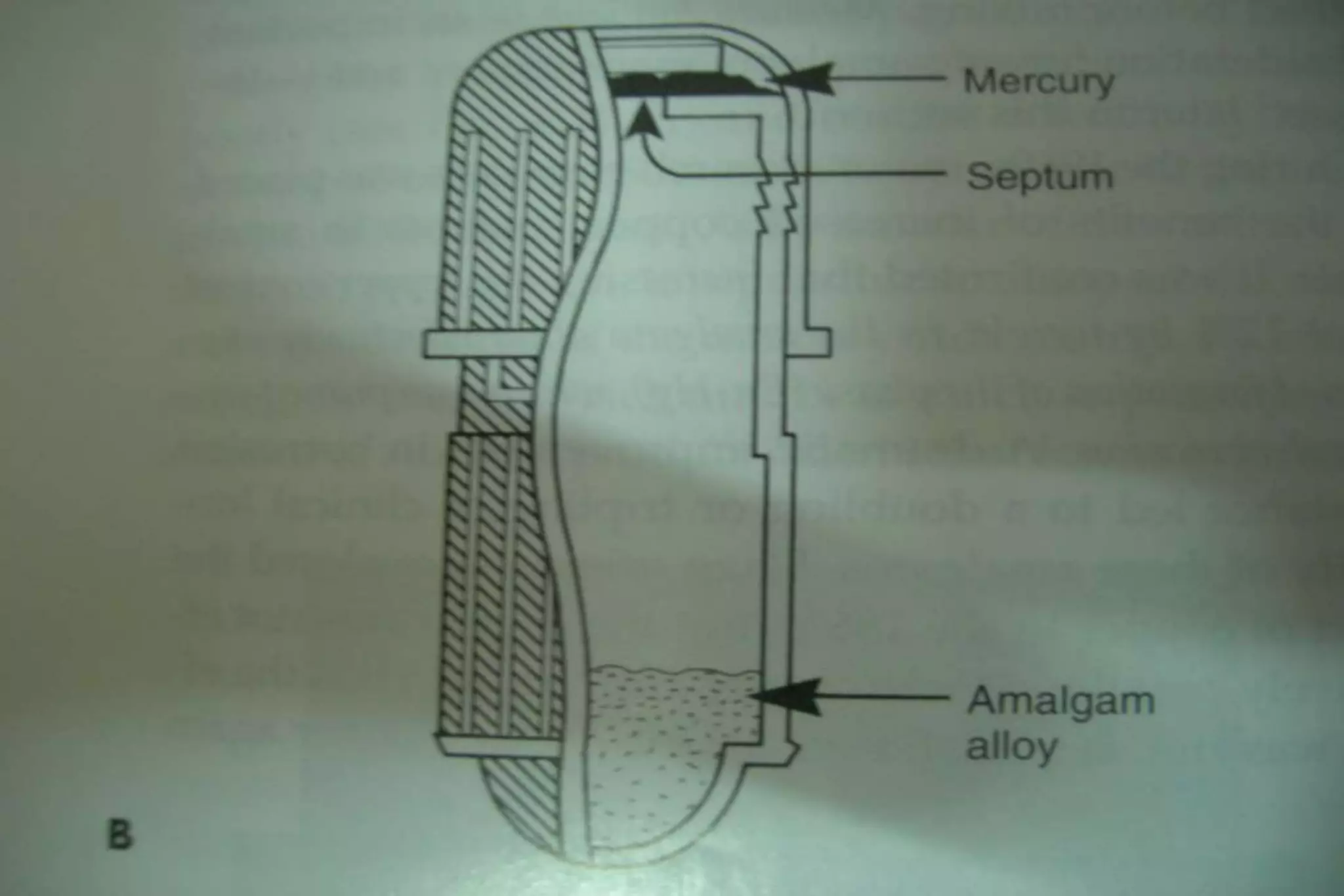
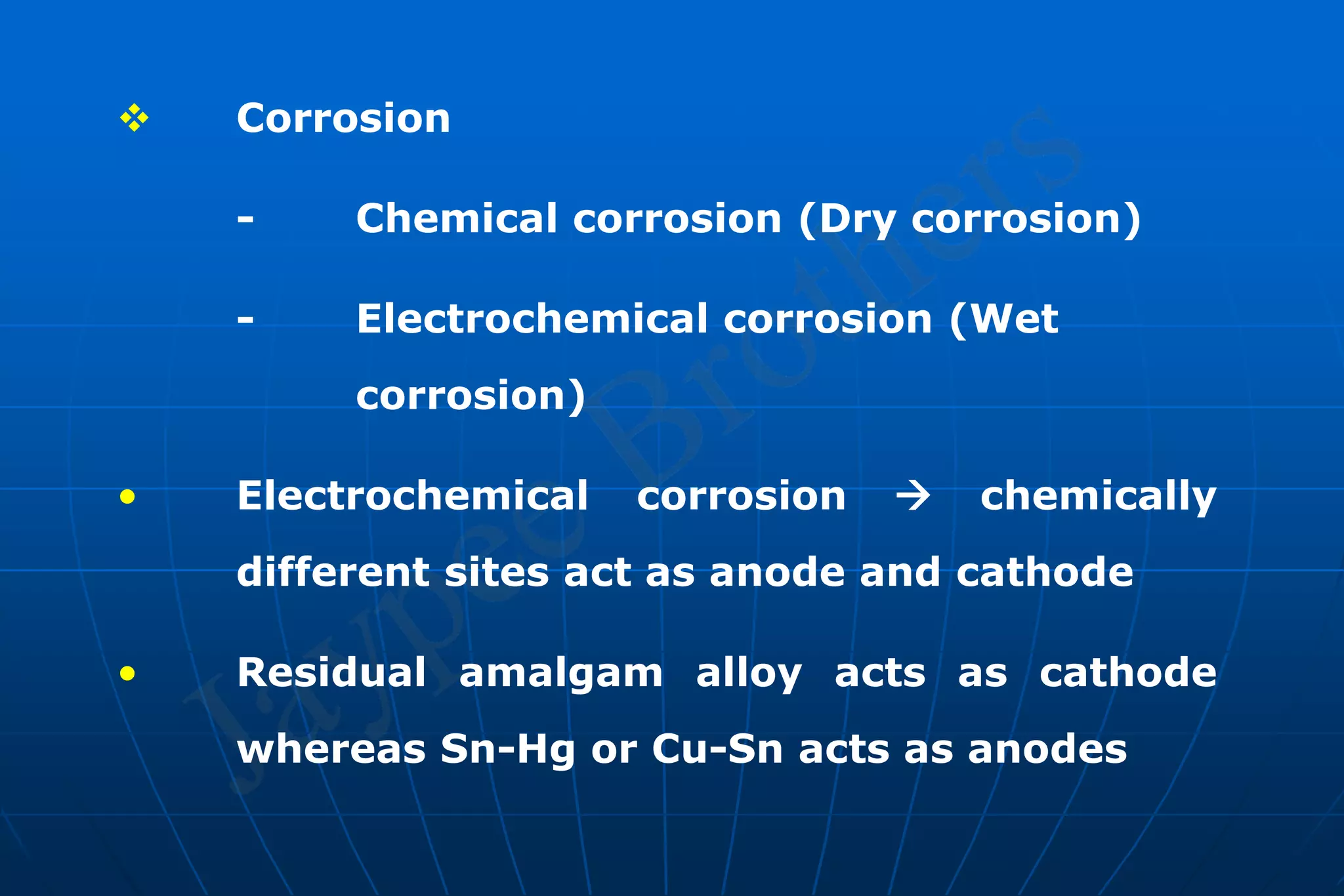
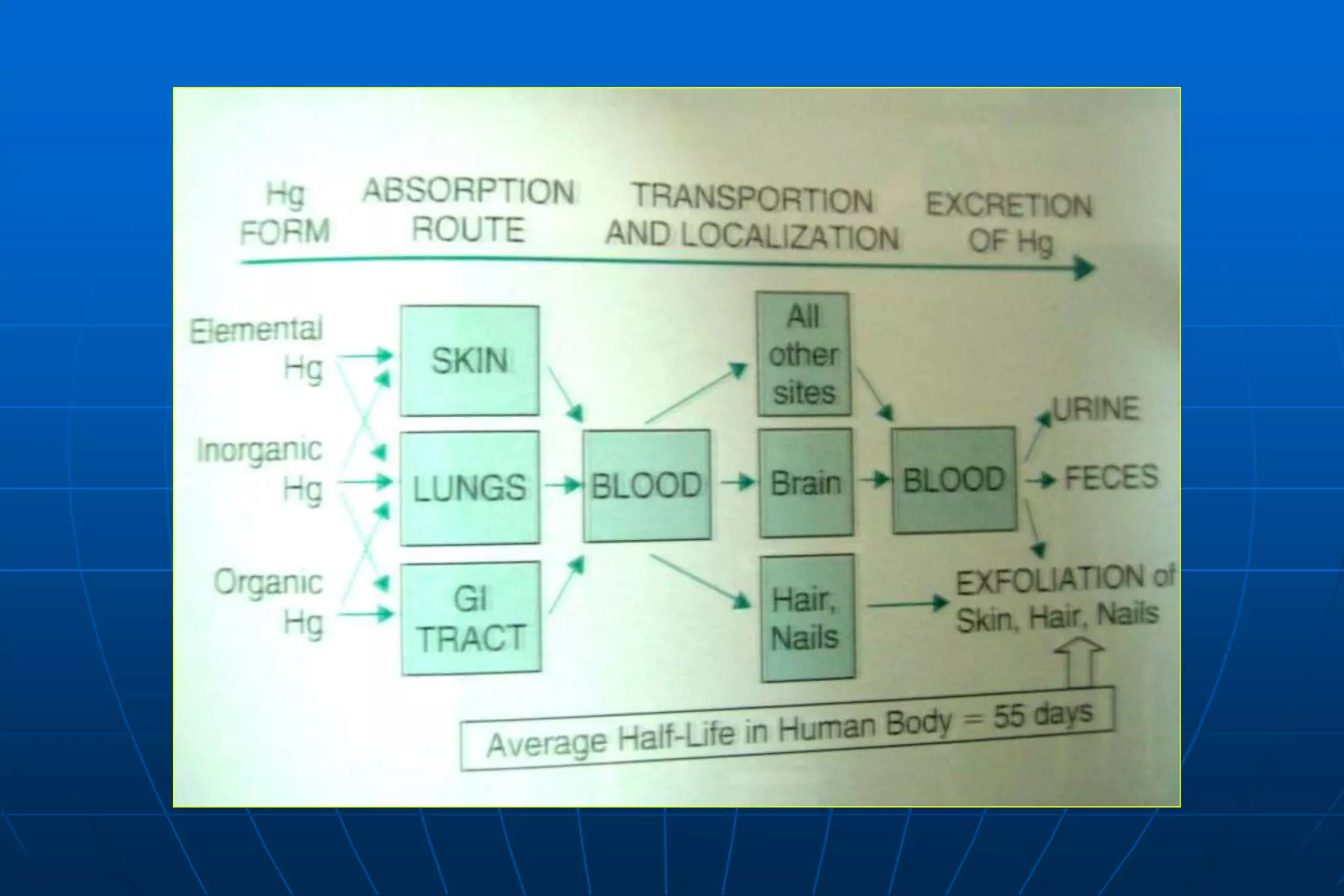
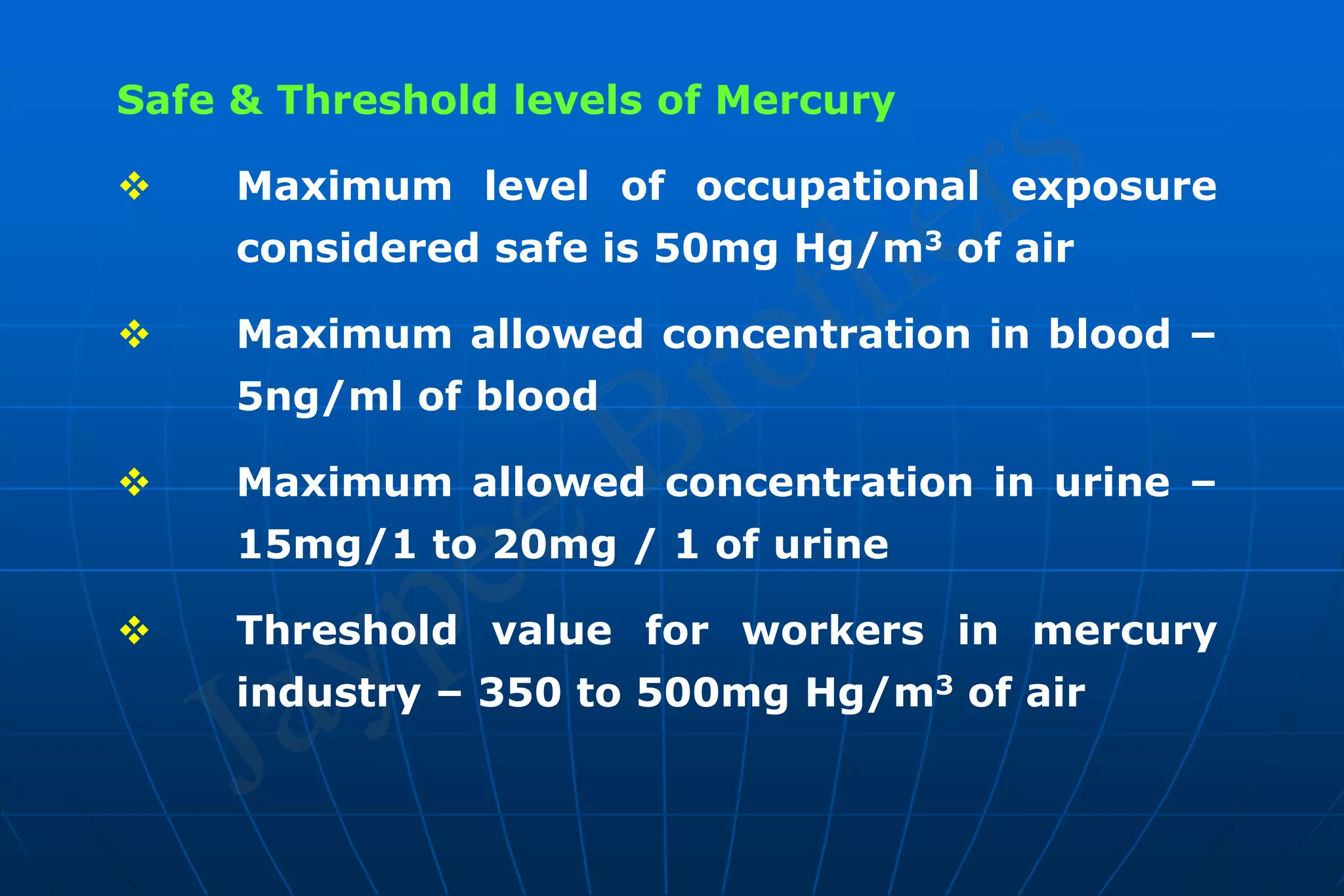
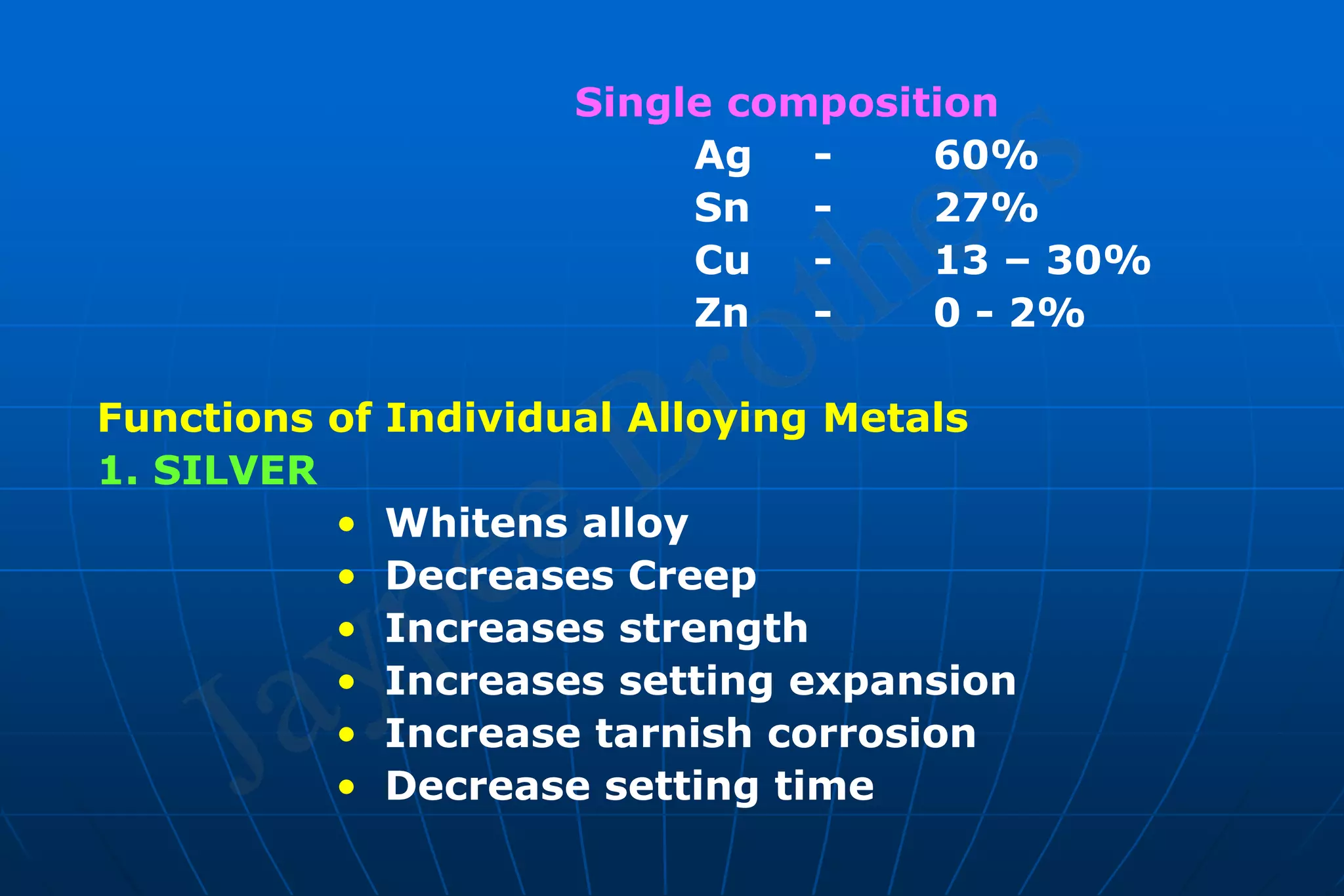
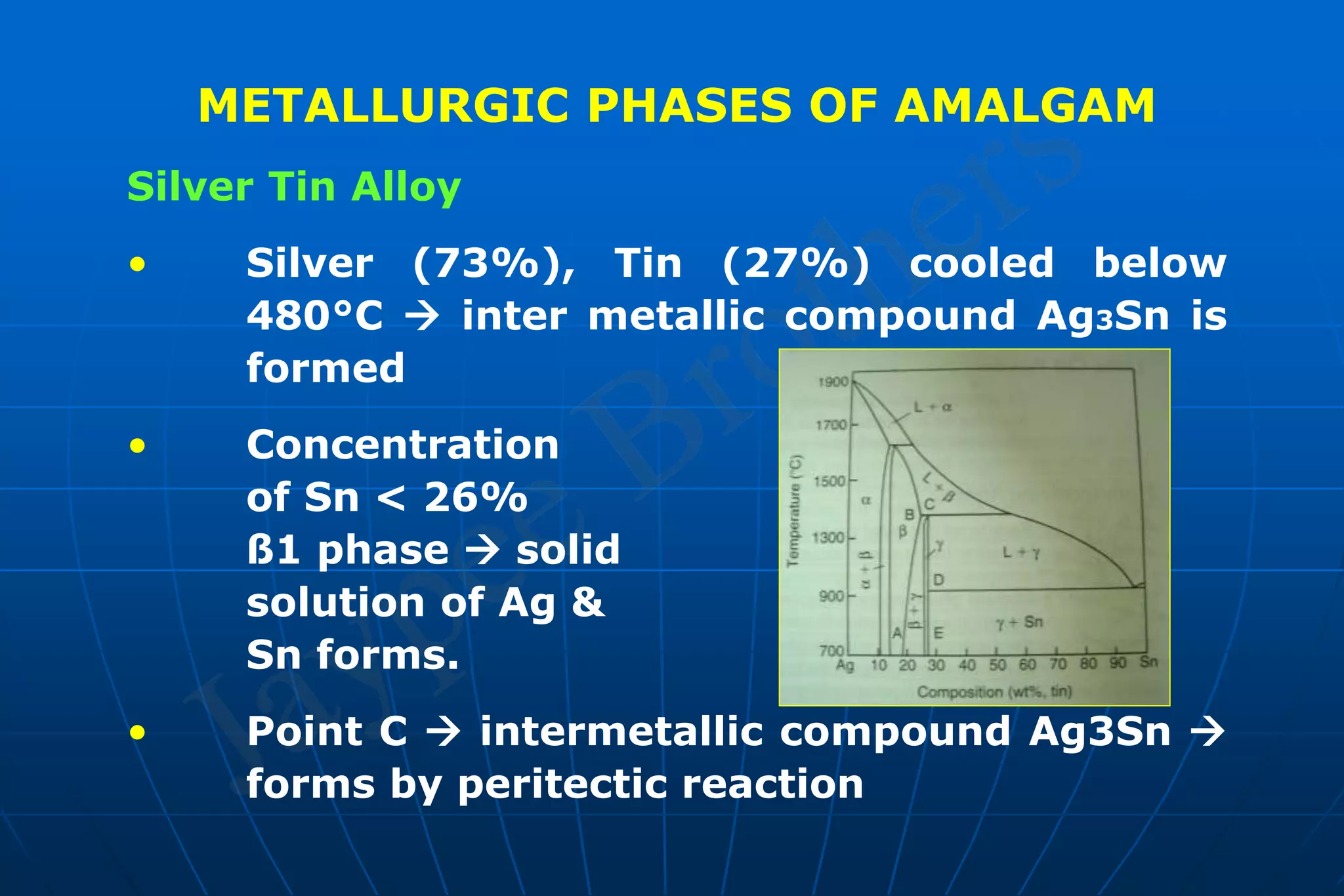
Dental amalgam is an alloy used in dental restorations that is a mixture of mercury and other metals such as silver, tin, and copper. It has been used in dentistry since the 1800s. The document discusses the history, composition, manufacturing process, setting reactions, properties and strengths of dental amalgam. It provides details on the phases that form during setting and how composition affects properties such as strength, expansion and corrosion resistance. High copper amalgams have higher strengths compared to low copper amalgams. Proper manipulation of the amalgam is important to achieve optimal strength.















































![- Reduce original Hg / alloy ratio (Eames
Tech/Minimal Hg Tech) [1:1]
• Sufficient Hg provides coherent
plastic mass
• Hg content of finished restoration
comparable to original Hg alloy
ratio
• Usually 50% with lesser amounts
with spherical alloys
• Technique reduces contact and
contamination with metallic Hg](https://image.slidesharecdn.com/dentalamalgam-221130051018-221e34c8/75/Dental-Amalgam-ppt-53-2048.jpg)