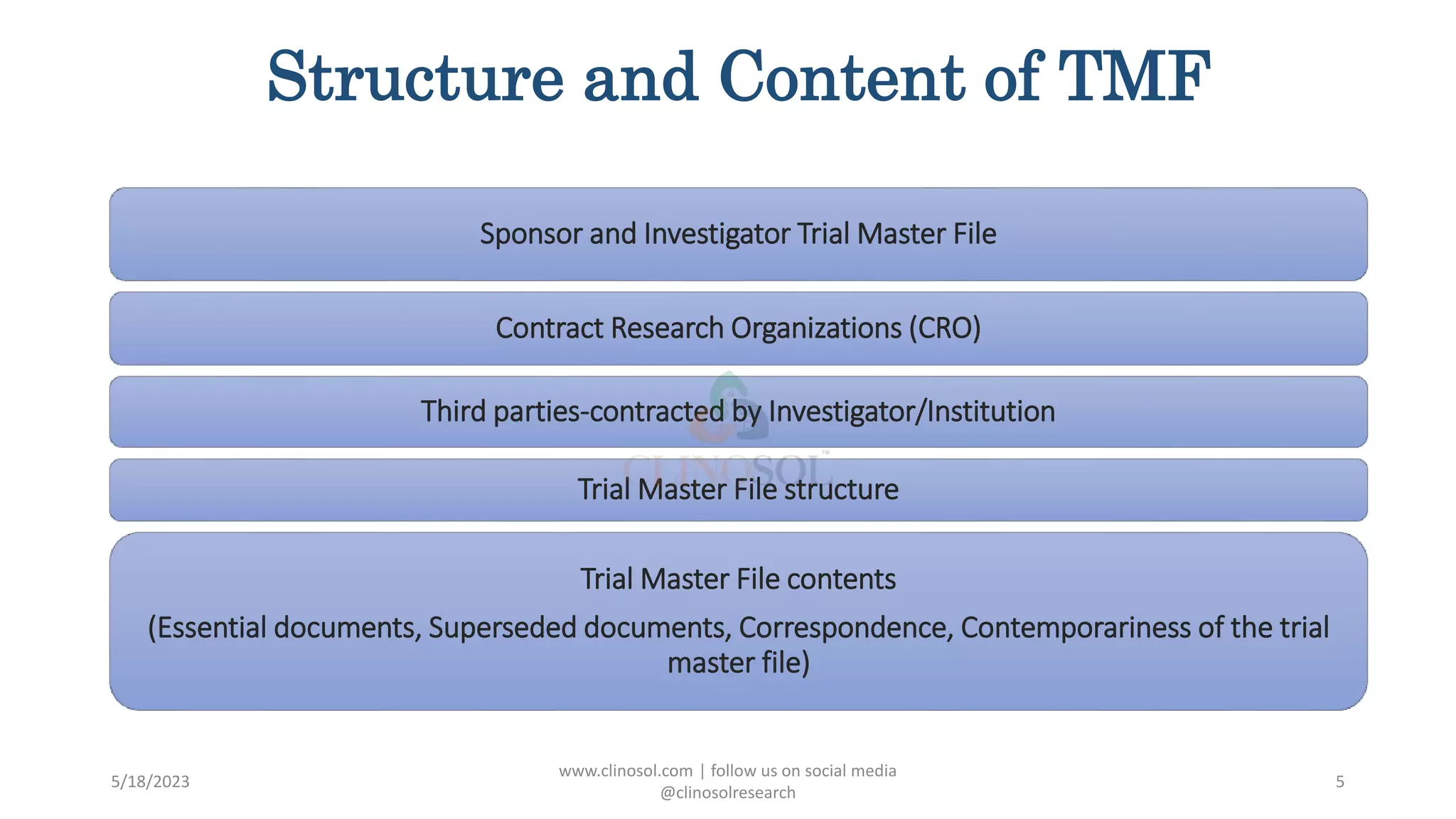
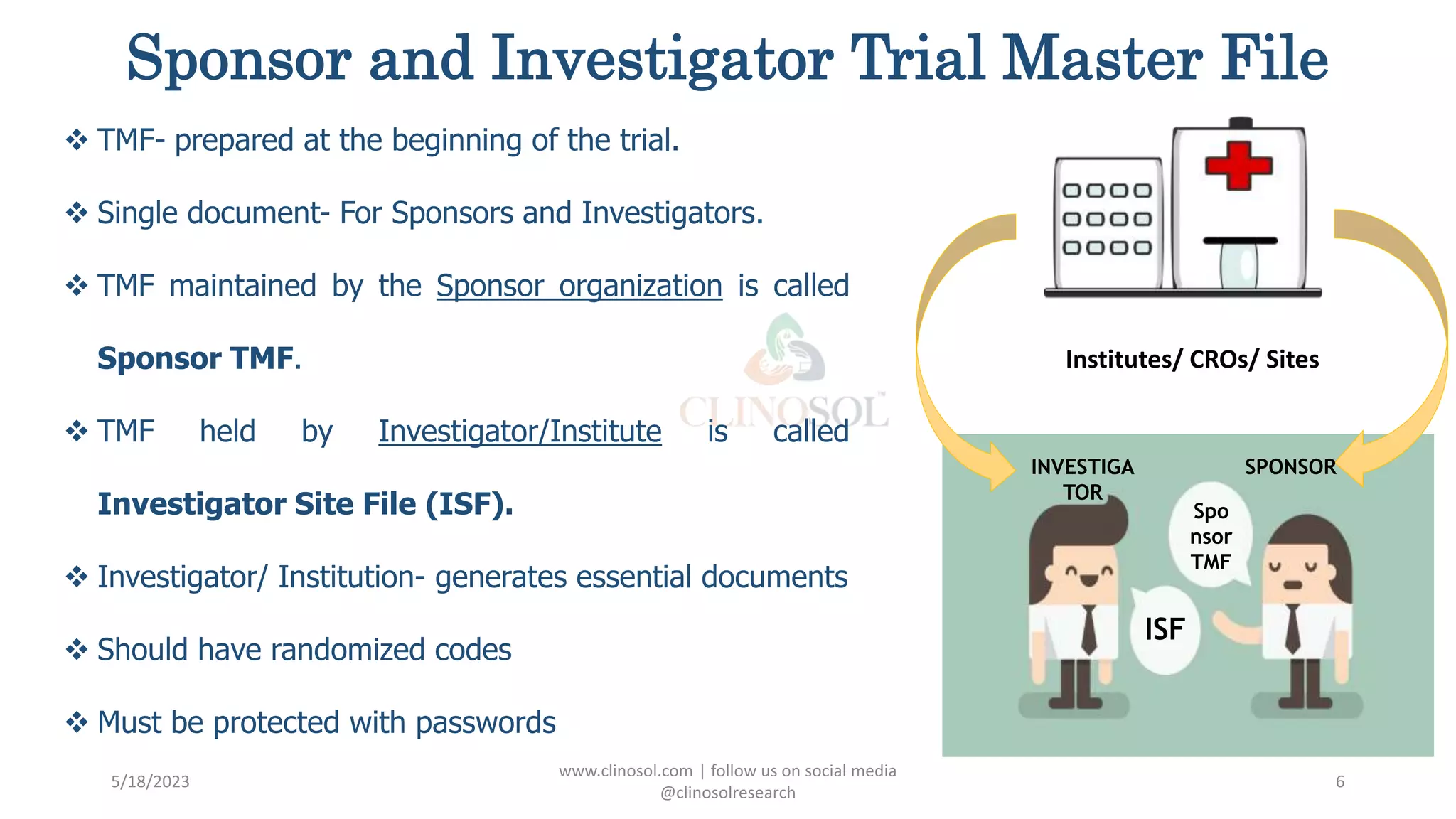
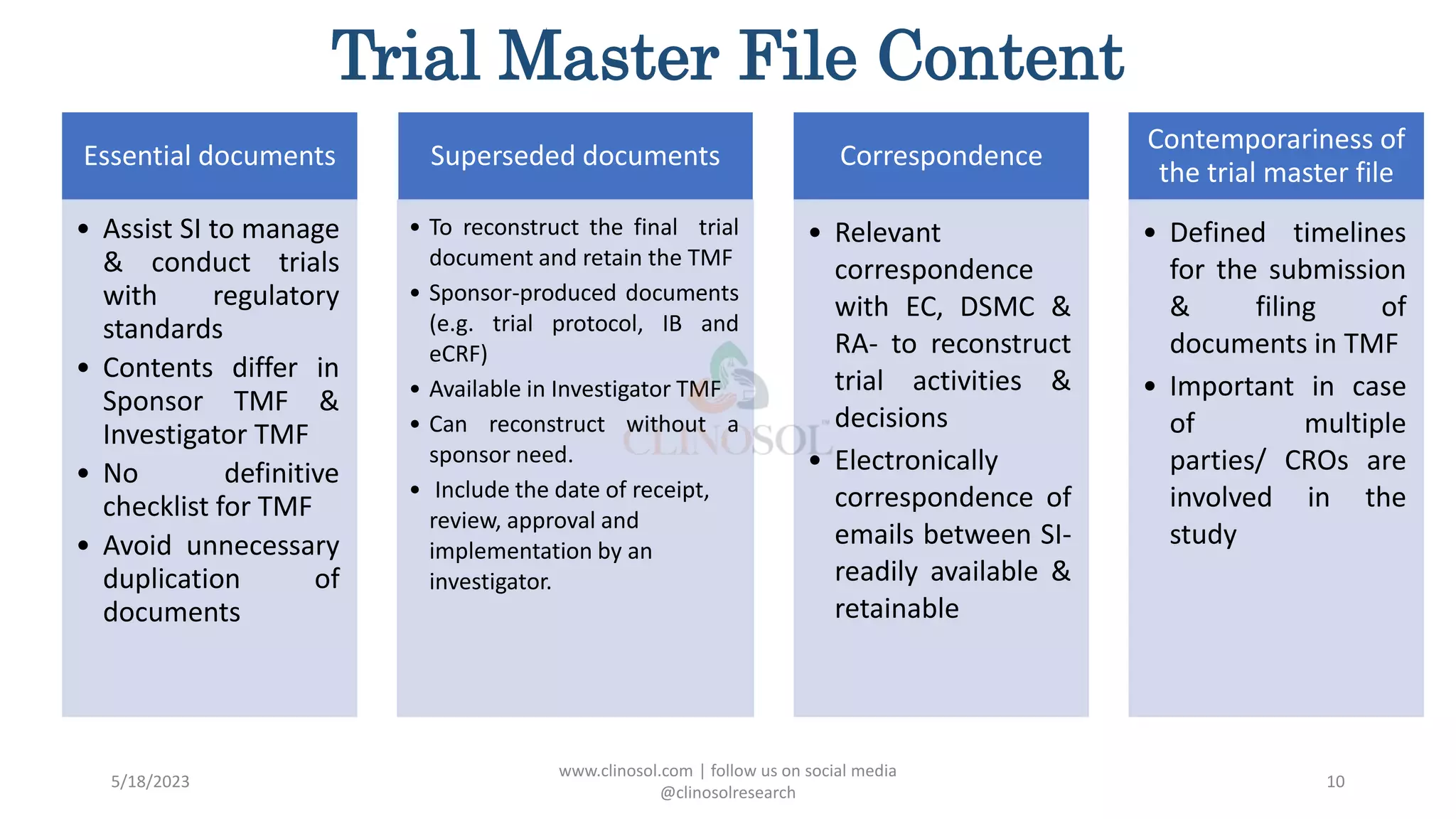
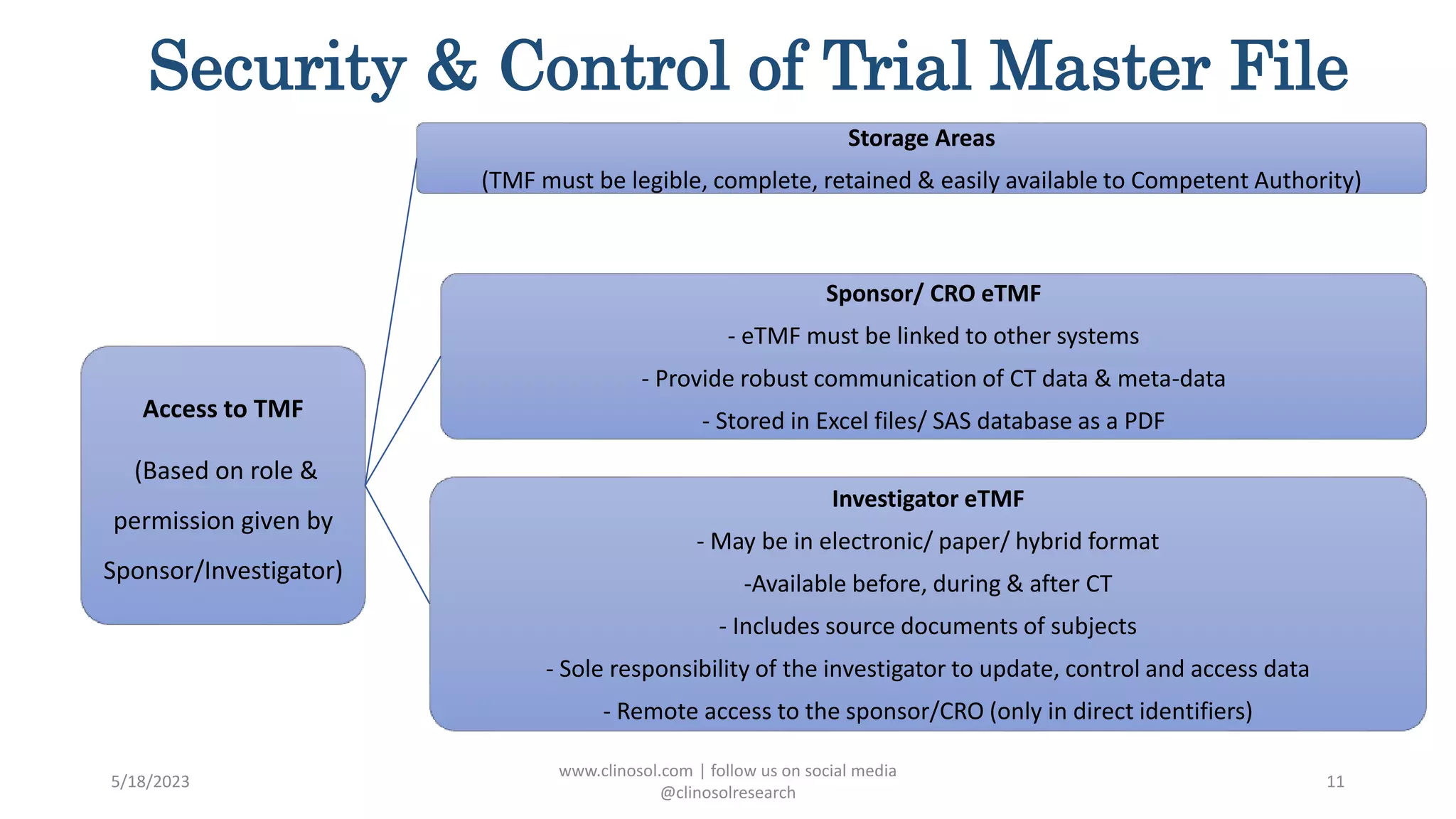
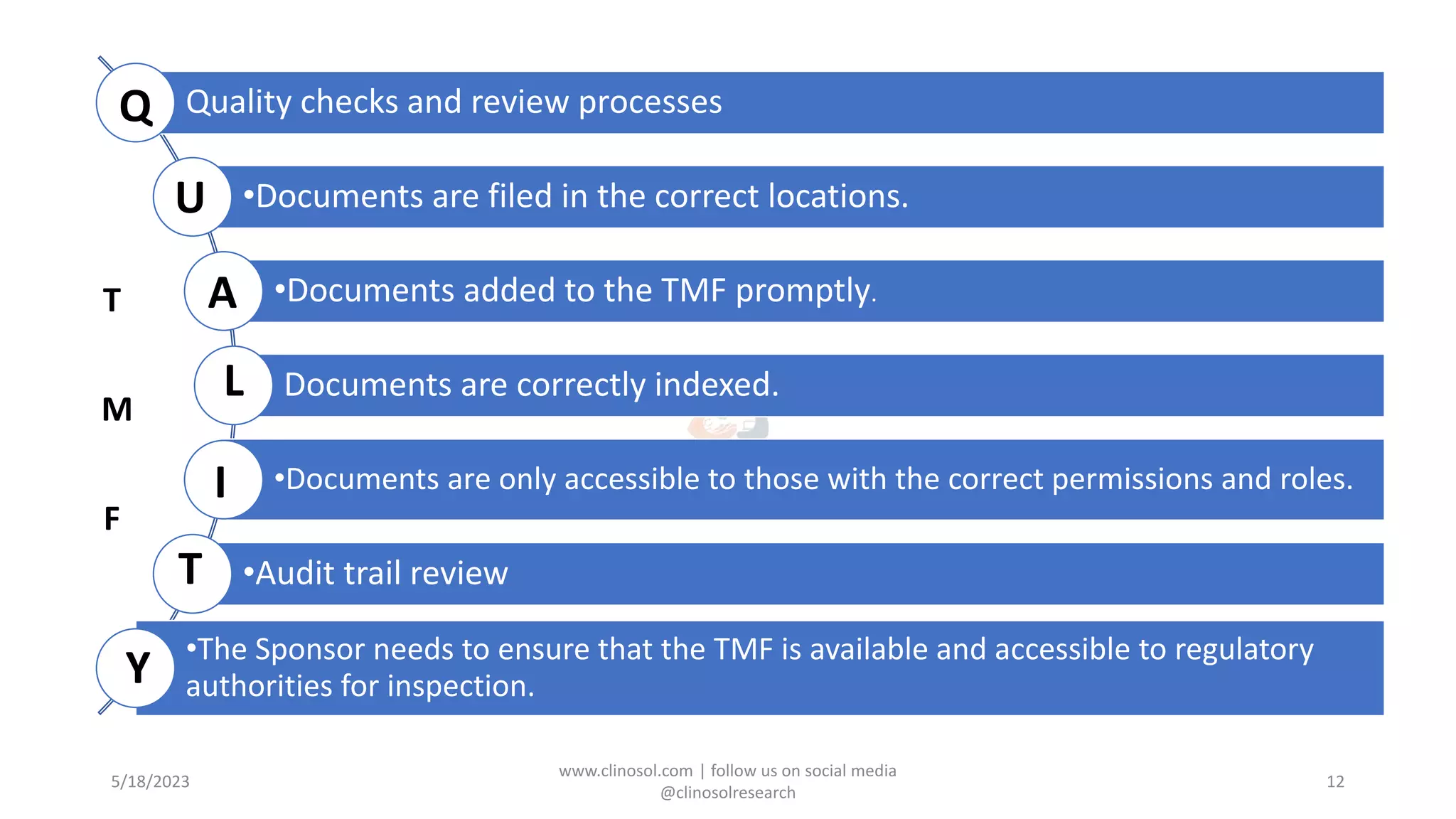
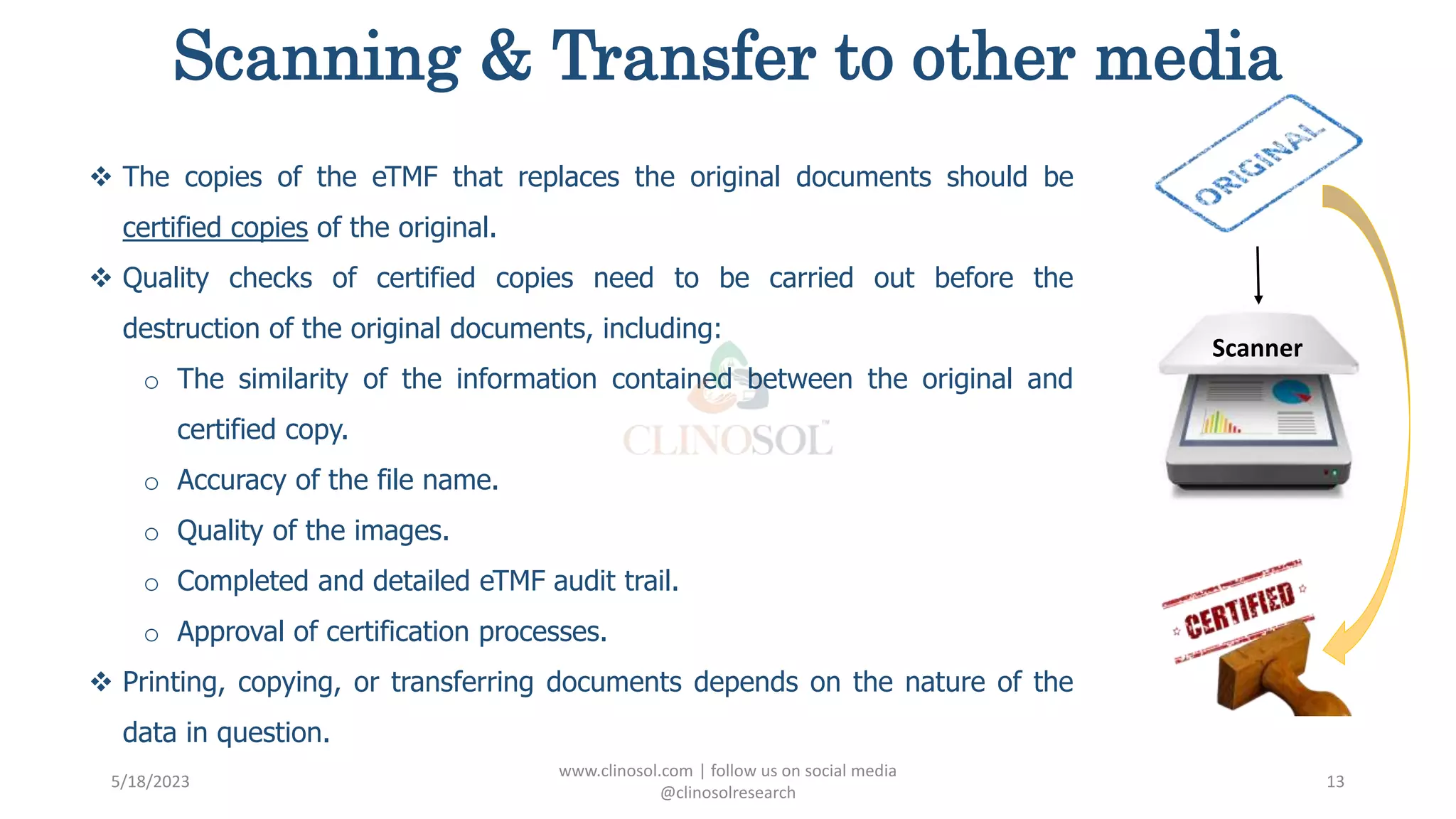
The document provides an overview of the Trial Master File (TMF) and Electronic Trial Master File (eTMF), which are essential collections of documents to evaluate clinical trial conduct and data credibility as per ICH-GCP guidelines. It details the structure, contents, and management of TMFs, emphasizing the transition from paper to digital formats, security, quality checks, archiving, and retention policies. The roles of sponsors, investigators, and contract research organizations (CROs) in maintaining the TMF are also outlined.