Embed presentation
Download as PDF, PPTX
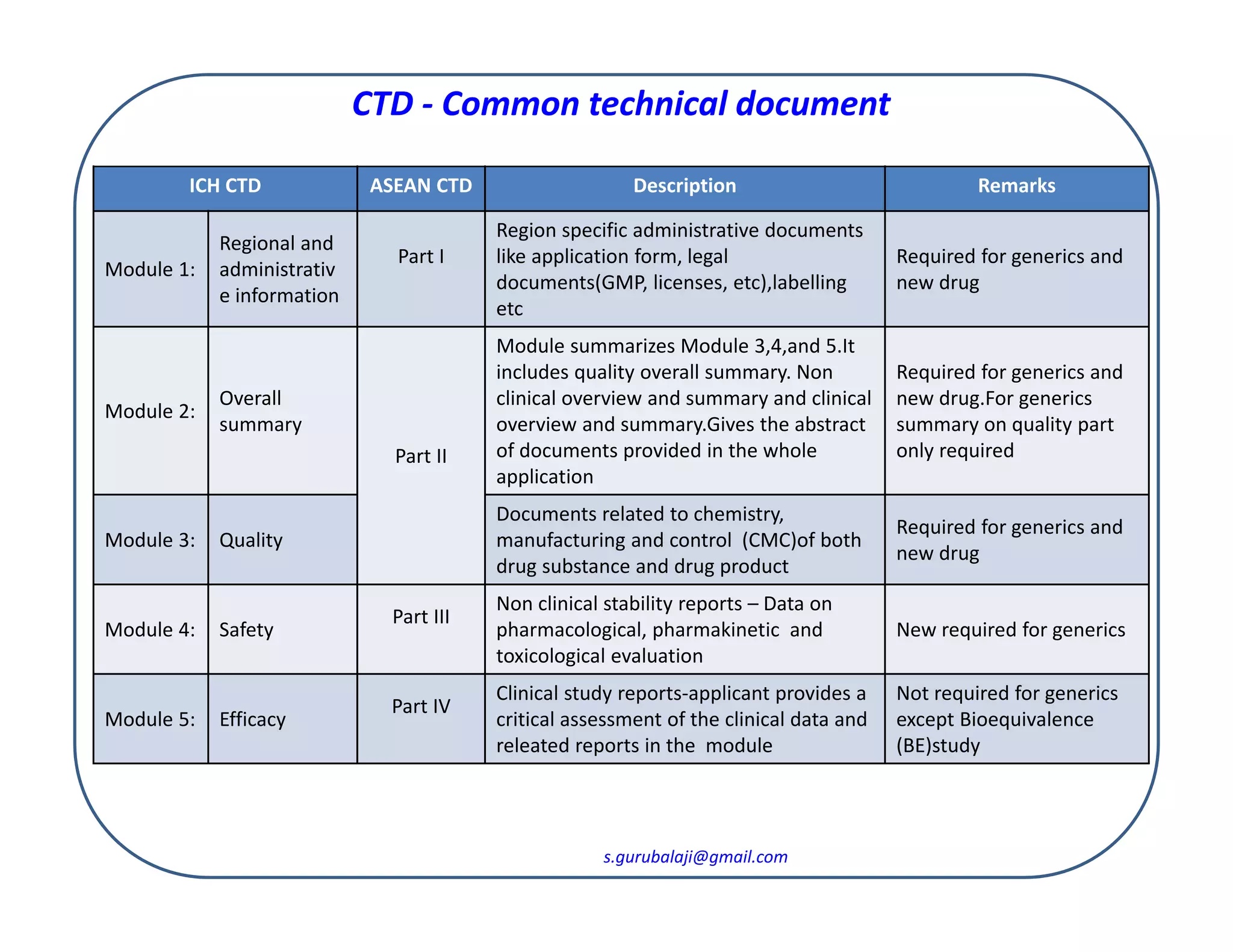

The document describes the five modules of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Common Technical Document (CTD) format for ASEAN countries. Module 1 contains regional administrative information. Module 2 provides an overall summary of Modules 3, 4, and 5, including quality, non-clinical, and clinical overviews. Module 3 includes chemistry, manufacturing, and quality control documents. Module 4 contains non-clinical safety data. Module 5 provides clinical study reports, though generics only need to include bioequivalence studies. The CTD format is required for both new drug and generic applications in ASEAN countries.
