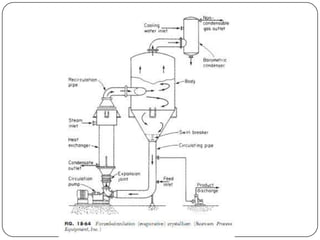
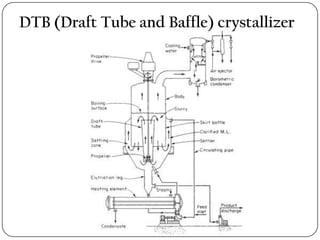
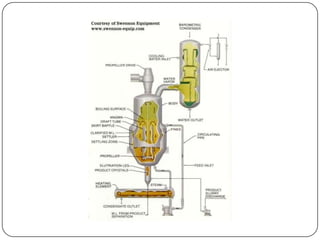
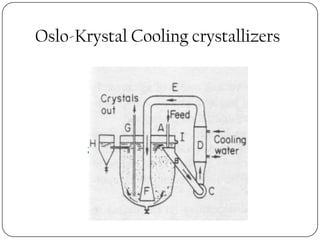
Crystallization and whole broth processing are important industrial techniques. Crystallization involves forming solid crystals from a solution, melt, or vapor and is widely used in pharmaceutical and chemical purification. It allows isolation of products with high purity at low cost. Whole broth processing recovers metabolites directly from unfiltered fermentation broth using methods like ion exchange resins, dialysis, expanded-bed adsorption, or resin absorption to minimize inhibitory effects during fermentation. Common equipment for crystallization includes tank crystallizers and forced circulation crystallizers.