This document provides information on diagnosing and differentially diagnosing COPD, including:
- Key indicators that should prompt consideration of a COPD diagnosis including dyspnea, chronic cough, sputum production, and risk factor exposure. Spirometry is required to confirm COPD.
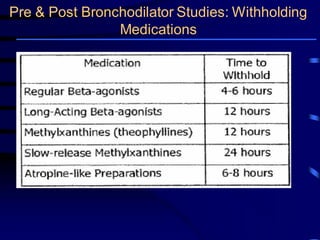
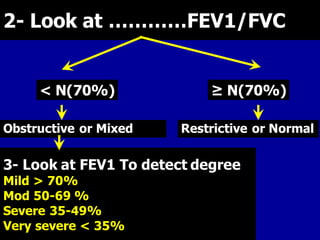
- Spirometry is the basic investigation needed to diagnose COPD. It assesses airflow limitation through FEV1/FVC ratio and severity through FEV1 levels. Reversibility testing can help differentiate COPD from asthma.
- Additional optional investigations that may be used include imaging like chest X-rays and CT scans to identify emphysema and airway abnormalities, lung volume measurements, diffusing capacity tests, and