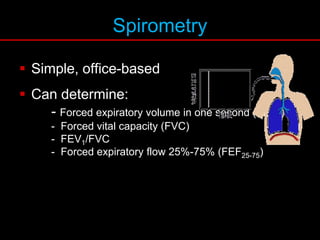
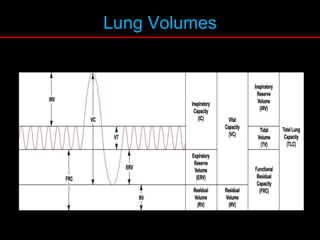
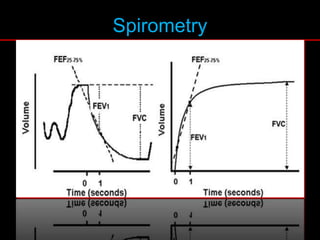
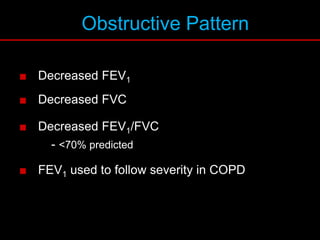
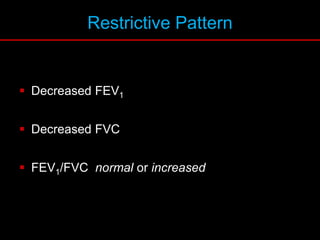
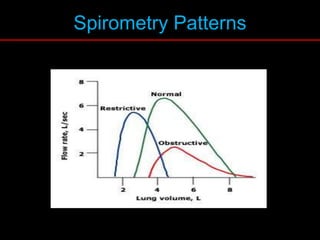
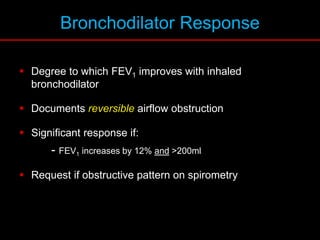
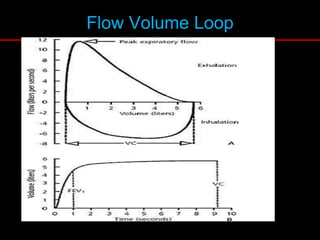
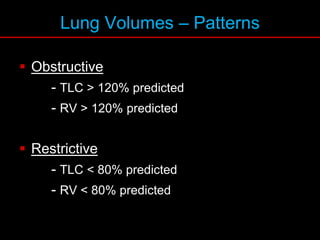
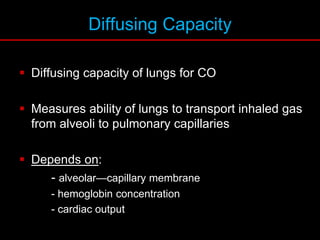
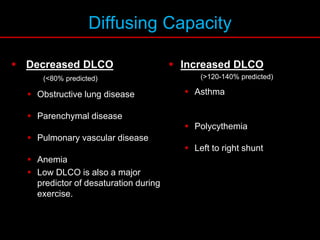
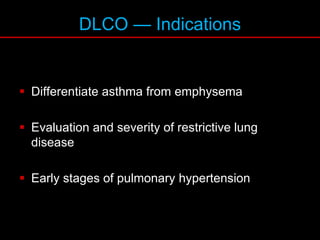
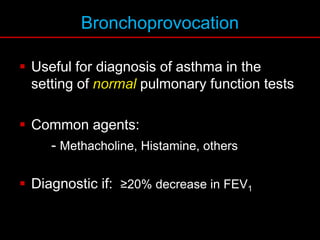
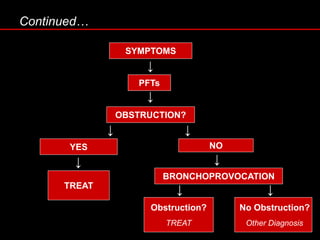
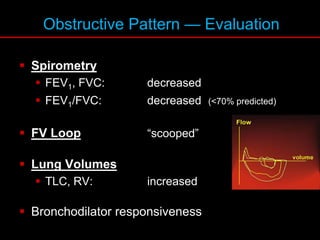
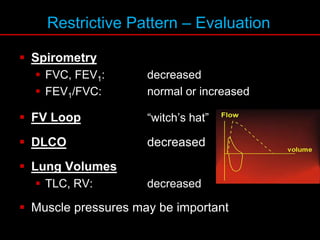
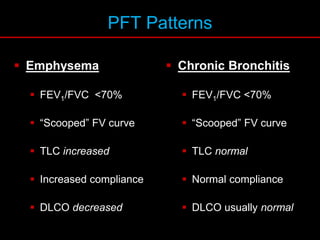
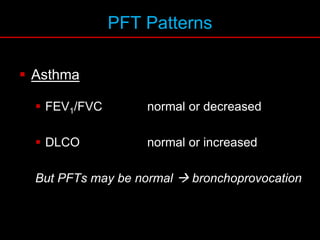
Pulmonary function tests (PFTs) measure how well the lungs work. Key PFT measurements include forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and their ratio (FEV1/FVC). PFTs can detect obstructive or restrictive lung patterns. In an obstructive pattern, FEV1 and FVC are reduced with a reduced FEV1/FVC ratio. A restrictive pattern shows reduced FEV1 and FVC but a normal or increased FEV1/FVC ratio. PFTs are used to diagnose and monitor lung diseases, evaluate patients for surgery, and guide treatment.