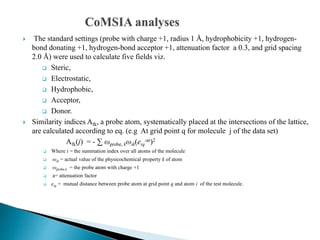
The document discusses 3D-QSAR studies conducted on a set of 52 carbamate compounds with acetylcholinesterase inhibitory activity. The compounds were divided into training and test sets. Comparative Molecular Field Analysis (CoMFA) and Comparative Molecular Similarity Indices Analysis (CoMSIA) models were developed using two alignment strategies: Maximum Common Substructure (MCS)-based alignment and pharmacophore-based alignment from Hip-Hop algorithm. The pharmacophore-based alignment produced superior models with predictive r2 values of 0.614 and 0.788 for CoMFA and CoMSIA respectively. Steric effects and hydrophobicity were found to play important roles in inhibitory activity. The studies suggest pharmac







![(A) Common feature-based (Catalyst/Hip-Hop) pharmacophore model (Hypo1) with distance
angular Å.
(B) mapping of the most active molecule [Hydrophobic (blue), hydrogen bond acceptor
(green), ring aromatic (yellow), and positive ionizable (red) and the distance between
chemical features are shown in Å].](https://image.slidesharecdn.com/rihanppt-161123053554/85/consensus-superiority-of-the-pharmacophore-based-alignment-over-maximum-common-substructure-11-320.jpg)



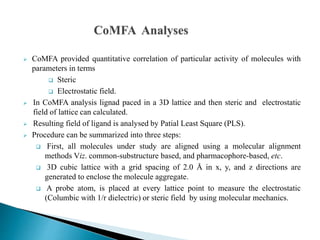




![ In the present study, an attempt has been made to correlate ache inhibitory activities of the
diverse carbamates with Steric,Electrostatic,Hydrophobic,Donor,Acceptor & field
descriptors.
The present study, where COMFA and COMSIA models based on pharmacophore-based
alignment are in good agreement with each other and demonstrated significant superiority.
The CoMFA and CoMSIA steric factor plays an important role as indicated by yellow map
suggesting that more and more bulk, and green map less bulk, respectively, are needed in this
region for enhancement of the activity.
The validation of the best CoMFA and CoMSIA models based on pharmacophore (hip-hop)-
based alignment on a test set of 17 compounds has provided significant predictive r2
[r2
pred(test)] of 0.614 and 0.788, respectively.
Hydrophobicity has been found to play a major role in the ache inhibitory activity
modulation.
The studies suggest that in the development of 3D-QSAR models, the hip-hop-based
alignment may be useful in getting predictive models which may provide useful information
required for proper understanding of the important structural and physicochemical features
for design-ing novel AChE inhibitors.](https://image.slidesharecdn.com/rihanppt-161123053554/85/consensus-superiority-of-the-pharmacophore-based-alignment-over-maximum-common-substructure-21-320.jpg)
