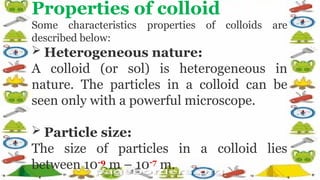
The document discusses colloids, highlighting their characteristics as heterogeneous mixtures with specific properties such as particle size, stability, and Brownian motion. Examples include milk, toothpaste, and blood, emphasizing that colloids cannot be separated by filtration and exhibit the Tyndall effect when light is scattered. Applications of colloids range from water purification to medicine and food production.